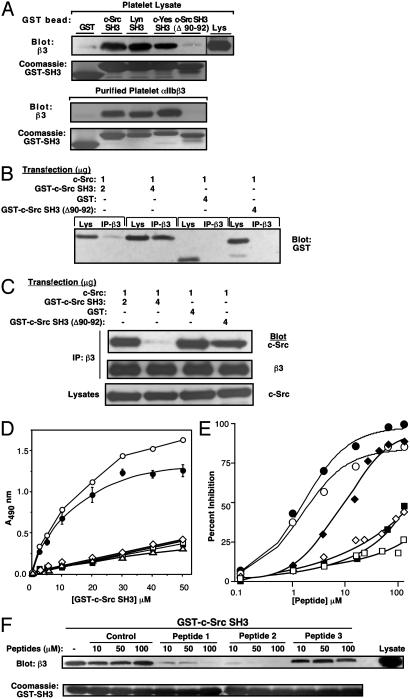
Fig. 3.
Interaction of c-Src SH3 with the integrin β3 cytoplasmic tail. (A) Platelet lysate or αIIbβ3 purified from platelets was incubated with glutathione-Sepharose beads coated with the indicated GST-SH3 domains. Bound proteins were eluted and probed with antibody to β3. (B and C) αIIbβ3 CHO cells were cotransfected with c-Src and the indicated GST fusion proteins. β3 immunoprecipitates were probed with antibody to GST (B), c-Src, or β3(C). (D) Direct binding of purified GST-c-Src SH3 to integrin β cytoplasmic tail proteins, assessed by ELISA: β3 (○), rβ3 (▪), β3(Δ758) (□), β1A (⋄), and β2 (▵). Specific binding of c-Src SH3 to β3(•). (E) Inhibition of the β3 tail/c-Src SH3 interaction by peptides. Immobilized β3 tail and soluble GST-c-Src SH3 (10 μM) were incubated with the following peptides: Src SH3-selective (LSSRPLPTLPSP) (•), Src family SH3-selective (KGGRSLRPLPPLPPPG) (○), β3 tail residues 722-741 (⋄), β3 748-762 (♦), αIIb 989-1008 (□), and control (KGELRLRNYYYDVV) (▪). Then, binding of GST-c-Src SH3 was detected by ELISA. (F) Inhibition of the αIIbβ3/c-Src SH3 interaction by peptides. Lysate from αIIbβ3 CHO cells was incubated with GST-c-Src SH3 coupled to beads in the presence of KGELRLRNYYYDVV (control), KGGRSLRPLPPLPPPG (peptide 1), LSSRPLPTLPSP (peptide 2), or APTYPPPLPP (peptide 3). β3 bound to beads was detected by immunoblotting.