Figure 2.
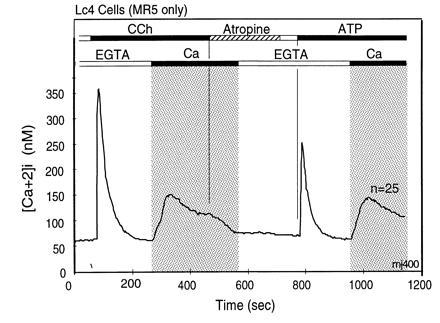
Dissociation of agonist-stimulated [Ca2+]i transients into IP3-induced release and CCE-mediated replenishment components. The first rapid rise does not require extracellular Ca2+ and returns rapidly to the starting [Ca2+]i, due to extrusion of Ca2+ from the cells by plasma membrane Ca-ATPases and reuptake of Ca2+ into stores by sarcoplasmic endoplasmic reticulum Ca-ATPases. The second component, stimulation of CCE, is seen as influx of Ca2+ from the extracellular milieu that occurs upon Ca2+ addition after completion of the first Ca2+ transient. This form of Ca2+ entry is due to stimulation of CCE channels by a signal generated by the IP3-induced store depletion and/or by some other agonist-induced signaling process. [Ca2+]i changes were measured in murine L cells (Lc4 cells) expressing in stable form the M5 muscarinic acetylcholine receptor. Bars on top depict changes in composition of the medium. The lower bars indicate Ca2+ additions; open bar indicates addition of 0.5 mM EGTA to medium without added Ca2+, and the black bar and gray area depicts change to a balanced salt solution containing 1.8 mM CaCl2. The upper bars indicate agonist additions. When added, concentration of CCh was 20 μM and that of ATP was 90 μM. Note that the initial rise of [Ca2+]i after agonist addition in the absence of extracellular Ca2+ returns spontaneously to baseline levels, and also note that addition of extracellular Ca2+ uncovers the presence of an activated influx pathway that causes a robust increase in [Ca2+]i. This influx is referred to as CCE. Note further that in these cells the release of Ca2+ from intracellular stores occurs as a concerted event without hint of oscillations.
