Figure 8.
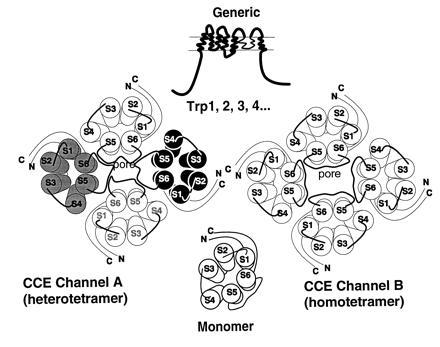
Models of secondary and quaternary structures of CCE channels and their subunits. CCE channels are hypothesized to be made of different Trps and/or different Trp combinations to account for CCE channels with varying ion selectivities and different forms of activation—e.g., store depletion-sensitive vs. store depletion-insensitive. The transmembrane topology chosen is arbitrarily based on that of voltage-gated K+, Na+, and Ca2+ channels and is thus far supported by the finding that hTrp3 is glycosylated and the results on HA localization in expression experiments. However, other transmembrane topologies are possible and the model may need major revisions. Existence of six nonallelic genes encoding six Trp-related proteins, opens the possibility that there are either diverse homomultimeric or diverse heteromultimeric CCE channels that could account for the functional heterogeneity of CCE seen in different tissues and cells.
