Figure 1.
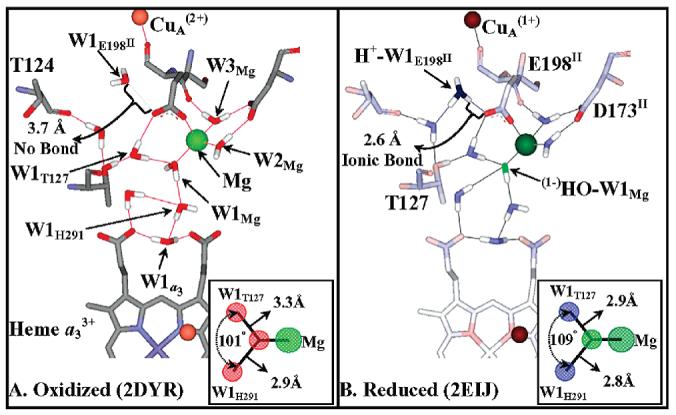
Formation of a hydroxide/hydronium ion pair upon CuA reduction, modeled on bovine oxidase oxidized and reduced structures. A comparison of the oxidized (A) and reduced (B) structures of bovine oxidase indicates a change in the protonation status and bonding of two water molecules. (i) W1E198II. W1E198II is HOH2289 in the bovine 2DYR (Ox) and HOH2291 in the bovine 2EIJ (Red) structures. The distances indicate that W1E198II switches between a neutral water molecule and a hydronium ion upon reduction. This switch in protonation status is accompanied by its movement of 1.1 Å toward the free carboxylate oxygen of E198II (OE2 in both structures). Upon CuA reduction H+−W1E198II becomes ionically bonded to the free carboxylate oxygen of E198II, the bond distance shortening from 3.7 to 2.6 Å. (ii) W1Mg. There are three waters bonded to the Mg ion, but only one can undergo deprotonation, W1Mg (HOH2032). W2Mg and W3Mg (HOH2031, HOH2033) are pinned by the Mg(II) ion and the carboxylate D173II (in the neutral state). A change in bond length between (1−)HO−W1Mg/W1Mg and the Mg(II) cation is not resolved in the bovine crystal structures; i.e., it is 2.3 Å in both structures. However, the bond lengths of W1Mg to the two nearest water molecules, W1H291 and W1T127 (HOH2020 and HOH2054), do show a considerable shrinkage upon reduction, 2.9 to 2.8 Å and 3.3 to 2.9 Å, respectively, due to increased ionic bonding character as W1Mg is converted to (1−)HO−W1Mg. The bond angles and bond lengths of W1Mg to W1H291 and W1T127 are shown in the insert of panels A and B.
