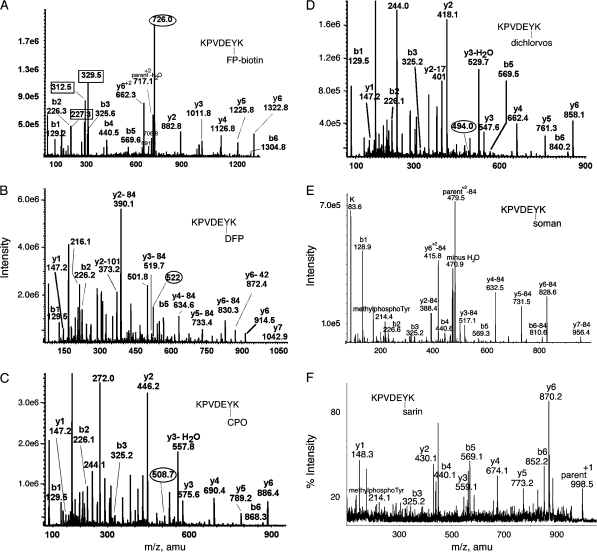
FIG. 4.
MS/MS spectra of OP labeled Tyr 238 in peptide KPVDEYK of human transferrin. Mass spectra in (A-D) were acquired on the QTRAP 4000 mass spectrometer by infusion, in panel E on the QTRAP 2000 by LC/MS/MS, and in panel F on the MALDI-TOF-TOF mass spectrometer. The b and y ion masses in all panels are consistent with OP covalently bound to Tyr 238. (A) The doubly charged parent ion of the FP-biotin–labeled peptide is at 726.0 m/z. Masses enclosed in boxes at 227.3, 312.5, and 329.5 amu are fragments of FP-biotin. The immonium ion of FP-biotinylated tyrosine is at 708.8 amu. Its partner ion at 691.6 amu has lost 17 amu. (B) The doubly charged parent ion of the DFP-labeled peptide is at 522.0 m/z. Loss of one or both isopropyl groups during collision-induced dissociation yields y-ions minus 42 or minus 84 amu, confirming the presence of diisopropylphosphate. Loss of both isopropyl groups is the most common observation (Grigoryan et al., 2008; Li et al., 2007). The y-ion series (y1 and y2–84 through y6-84) indicates that tyrosine is labeled. The delta mass (242.9 amu) between y1 (147.2 amu) and y2–84 (390.1 amu) is consistent with the appearance of tyrosine phosphate at fragment y2 (163 amu for tyrosine and 80 amu for phosphate). The mass at 373.2 amu is the y2 ion minus 101 amu, representing loss of two isopropyl groups as well as ammonia. The mass at 501.8 m/z is the doubly charged parent ion minus one isopropyl group. The mass at 216.1 amu is consistent with the phosphotyrosine immonium ion. (C) The doubly charged parent ion of the chlorpyrifos oxon-labeled peptide is at 508.7 m/z. The y-ion series (y1–y6) shows the presence of all residues. The mass difference (299.0 amu) between y1 (147.2 amu) and y2 (446.2 amu) clearly shows the diethoxyphosphate on tyrosine in fragment y2 (163 amu for tyrosine and 136 amu for diethoxyphosphate). The mass at 244.1 amu is consistent with the monoethoxyphosphotyrosine immonium ion. The mass at 272.0 amu is consistent with the diethoxyphosphotyrosine immonium ion. (D) The doubly charged parent ion of the dichlorvos-labeled peptide is at 494.0 m/z. The y-ion series (y1–y6) shows the presence of all residues. The mass difference (270.9 amu) between y1 (147.2 amu) and y2 (418.1 amu) clearly shows the dimethoxyphosphate on tyrosine in fragment y2 (163 amu for tyrosine and 108 amu for dimethoxyphosphate). The mass at 244.0 amu is consistent with dimethoxyphosphotyrosine immonium ion. (E) The parent ion of the soman-labeled peptide has a mass to charge ratio of 520.7, but this mass does not appear in the scan. The prominent peak at 479.5 is the doubly charged parent ion that has lost the pinacolyl group from soman. The y2–y7 ions have all lost 84 amu due to release of pinacolyl from soman. The peak at 214.4 is the methylphosphotyrosine immonium ion. (F) The parent ion of the sarin-labeled peptide is the singly charged ion at 998.5 amu. The masses of the y-ion series, and the 214.1 mass for methylphosphotyrosine immonium, support labeling on tyrosine.