Abstract
Lymphocytes require glucose uptake and metabolism for normal survival and function. The signals that regulate the expression and localization of glucose transporter 1 (Glut1) to allow glucose uptake in T cells are now beginning to be understood. Resting T cells require extracellular signals, such as cytokines, hormones, and growth factors, or low-level TCR stimulation to take up adequate glucose to maintain housekeeping functions. In the absence of extrinsic signals, resting T cells internalize and degrade Glut1 and cannot maintain viability. Activated T cells have dramatically increased metabolic requirements to support the energy and biosynthetic needs necessary for growth, proliferation, and effector function. In particular, glucose metabolism and aerobic glycolysis fuel this demand. Therefore, activation of T cells causes a large increase in Glut1 expression and surface localization. If glucose uptake is limited, glycolytic flux decreases to a level that no longer sustains viability, and proapoptotic Bcl-2 family members become activated, promoting cell death. However, excessive glucose uptake can promote hyperactive immune responses and possible immune pathology. Tight regulation of glucose uptake is required to maintain immune homeostasis, and understanding of these metabolic pathways may lead to therapeutic strategies to target some forms of cancer or autoimmunity.
Keywords: aerobic glycolysis, Glut1, Akt, IL-7, CD28
INTRODUCTION
The immune system is crucial for the defense against organisms that cause infections and against toxic products that may be released from the infectious agents. A functional immune response requires rapid and extensive cell growth, proliferation, and production of effector proteins. A defect in any single component of the immune system can cause a breakdown in this defense and may lead to serious or fatal diseases such as infections, cancers, or autoimmune disorders. In addition, a growing body of evidence suggests that excess inflammation decreases longevity [1,2,3,4]; therefore, fine regulation of this system is required to maintain health. Lymphocytes are part of the adaptive immune response and as such, are crucial for normal immune functions. T or B cell deficiencies are known to result in severe immunodeficiencies [5]. For that reason, it is vitally important to understand how normal lymphocyte function is regulated and fueled to allow energy and biosynthetic precursors for lymphocyte growth and effector function. To that end, this review will discuss the regulation of lymphocyte metabolism and the consequences of disrupting normal metabolism in these cells.
LYMPHOCYTE METABOLISM
T cells use glucose and glutamine as their primary fuel source [6], although ketone bodies and fatty acids can also be used to a small degree. Of these nutrients, glucose appears to be particularly necessary for cell survival, size, activation, and cytokine production [7]. Glucose provides much-needed energy for the lymphocyte in the following ways: Glucose can serve as a primary substrate for the generation of ATP; glucose can supply a carbon source for the synthesis of other macronutrients, such as nucleic acids and phospholipids; and glucose can be metabolized by the pentose phosphate pathway to generate NADPH.
For ATP generation, glucose can be metabolized via glycolysis or oxidative phosphorylation [7]. Glycolysis occurs in the cytosol, where one molecule of glucose is broken down into two molecules of pyruvate. The net reaction is oxygen-independent and yields two molecules of ATP for every one molecule of glucose. Pyruvate is then converted to lactate and generates electron acceptor NAD from NADH. Alternatively, oxidative phosphorylation is oxygen-dependent and occurs within the mitochondria. It is comprised of two reactions: conversion of intermediate molecules (pyruvate and fatty acids) to acetyl coenzyme A (coA) and degradation of acetyl coA to CO2 in the tricarboxylic acid cycle, yielding free electrons carried by NADH and flavin adenine dinucleotide (FADH2), as well as transfer of electrons from NADH and FADH2 to the electron transport chain, resulting in protons moving out of the mitochondrial matrix. The electrochemical potential is then used by ATP synthase to make ATP, with a total yield of 30 molecules of ATP from each molecule of glucose. Thus, oxidative phosphorylation is a seemingly more efficient way to generate ATP from glucose, although few metabolites remain for biosynthesis.
Resting lymphocytes have low-energy needs and derive most of their ATP from oxidative phosphorylation [8]; however, activated lymphocytes require a dramatic increase in metabolism upon activation. This is necessary to produce the energy required to stimulate growth and proliferation and produce the protein products expressed by activated immune cells [6, 7, 9, 10]. Glucose metabolism changes by orders of magnitude in an activated T cell, and the transition from a resting to an activated T cell causes a switch from catabolic to anabolic metabolism, in which ATP is used to produce complex macromolecules from simpler intermediates [11].
Activated lymphocytes generate energy in large part by up-regulating aerobic glycolysis [6, 7, 9], which describes the metabolic program used when a cell continues to convert pyruvate to lactate, despite conditions of adequate oxygen; it is used by many types of transformed or cancer cells [12, 13]. This shared metabolic program between activated lymphocytes and cancer cells further deepens the relevance of studying lymphocyte metabolism. It is not completely clear why an activated T cell chooses to use aerobic glycolysis for energy generation, although it may be that the rapid speed of glycolysis and the availability of biosynthetic precursors may favor glycolysis, despite the seemingly higher ATP production of oxidative metabolism. Regardless, a failure to increase glucose metabolism during lymphocyte activation prevents cell growth [6, 7, 9, 10, 14].
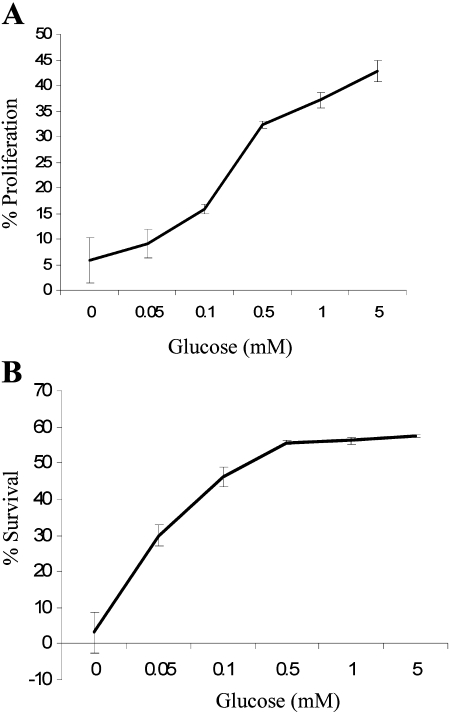
We have shown that even in the presence of excess, alternative energy sources, glucose limitation can prevent T cell proliferation and survival [15]. To measure the proliferation of T cells in response to limited glucose, T cells were labeled with CFSE and then incubated on plates coated with anti-CD3 and anti-CD28 for 48 h in media with limited glucose concentration (0–5 mM). T cells can use glucose or glutamine as a carbon source [6], and so, media did contain ample glutamine as an alternative carbon source. The CFSE signal is divided among daughter cells following cell division. Proliferation was therefore measured by quantifying the amount of CFSE fluorescence in each individual cell. CD3/CD28-stimulated T cells had increased proliferation in response to increasing concentration of glucose (Fig. 1A), which is therefore a preferred and essential nutrient for T cell activation. In addition, glucose is essential to promote T cell survival. To measure the survival of T cells in response to limited glucose, T cells were incubated on plates coated with anti-CD3 and anti-CD28 for 48 h in limited glucose concentration (0–5 mM), and cell survival was analyzed flow cytometrically by propidium iodide exclusion. Activated T cells had an increase in survival in a glucose concentration-dependent manner (Fig. 1B).
Fig. 1.
T cells require glucose for proliferation and survival. T cells were cultured in limited glucose concentration on plates coated with 5 μg/ml anti-CD3 and 5 μg/ml anti-CD28 for 48 h. (A) Cell proliferation was measured by CFSE staining, and the percentage of cells that divided at least twice was quantified. (B) Cell survival was measured flow cytometrically by propidium iodide exclusion.
Together, these data show that activated T cells require glucose for proliferation and survival; in the absence of glucose, T cells will not proliferate, even if adequate glutamine is present. Protein synthesis during lymphocyte growth also depends on glucose metabolism for ATP and biosynthetic substrates, and thus, cells deprived of adequate glucose levels cannot produce the immune products required for effector function, such as IFN-γ [8, 9, 16, 17]. Therefore, the growth, function, and survival of an activated lymphocyte depend on a dramatic increase in glucose metabolism that is not simply responsive to energy demands but is directly regulated and has a profound impact on T cell survival and function.
GLUCOSE TRANSPORTERS
Glucose uptake is controlled in part by the cell-surface expression of a family of glucose transporters [18, 19]. The best-known example in this family is glucose transporter 4 (Glut4), which is expressed in muscle, liver, and adipose tissue, and whose surface expression is up-regulated by insulin receptor activation [20,21,22]. Lymphocytes, however, do not express Glut4 and instead, rely primarily on the surface expression of Glut1, a ubiquitously expressed glucose transporter [9, 23]. Glut1 has been found to be overexpressed in many cancers [24], and interestingly, Glut1 is a coreceptor for the human T cell leukemia virus [25].
Glut1 expression must be properly controlled for immunity and to prevent autoimmunity. In the past, little has been known about Glut1 regulation or its role in T cell glucose metabolism. Our laboratory has studied the regulation of this glucose transporter in lymphocytes and hematopoietic cell lines. Not surprisingly, there are several signals and stimuli that can promote Glut1 expression and cell-surface trafficking and thereby, increase glucose in the T cell, including TCR stimulation, cytokine/growth factor stimulation, and hormones. In the absence of such signals, glycolytic flux decreases to a level that no longer sustains viability, and proapoptotic Bcl-2 family members become activated, promoting cell death [26].
Glut1 TRAFFICKING
Glucose uptake can be modulated at multiple points in addition to Glut1 expression, including transport activity [27, 28] and localization to the cell surface [29, 30]. Using hematopoietic cell lines that are dependent on IL-3 for survival, we and others have demonstrated that Glut1 trafficking is a regulated process and that IL-3 treatment maintained surface levels of Glut1 and promoted recycling of intracellular Glut1 [29,30,31]. Specifically, when cells were withdrawn from cytokine, Glut1 was internalized, and upon readdition of cytokine, Glut1 was returned back to the cell surface. Inhibition of new protein synthesis with cyclohexamide did not prevent the return of Glut1 to the cell surface, indicating that surface Glut1 was recycled from intracellular stores and trafficked to the cell surface. Furthermore, IL-3 attenuated Glut1 internalization and promoted Rab11a-dependent recycling of intracellular Glut1 [30].
The PI-3K/Akt signaling pathway in particular plays a critical role in cytokine-regulated Glut1 trafficking [30]. Inhibition of PI-3K activity by use of the pharmacologic inhibitor LY294002 decreased the ability of IL-3/cytokine to increase surface Glut1 levels. Furthermore, overexpression of a constitutively active form of Akt (myristoylated Akt) was sufficient to maintain surface Glut1 in the absence of IL-3/cytokine signal [30].
We further examined signals downstream of Akt that may be important in maintaining surface Glut1. We demonstrated that Akt did not require mammalian target of rapamycin (mTOR) to maintain surface levels, although inhibition of mTOR by rapamycin greatly diminished glucose uptake, suggesting that Akt-stimulated mTOR activity may promote Glut1 activity, if not trafficking [30]. Alternatively, inhibition of glycogen synthase kinase-3 (GSK-3) by a pharmacologic inhibitor SB216763 partially maintained surface Glut1 levels in the absence of IL-3/cytokine. Maintenance of Glut1 cell-surface levels was likely a result of increased recycling of Glut1 via inactivation of GSK-3 by Akt, as Glut1 internalization was unaffected by GSK-3 inhibition [30]. Thus, Akt inactivation of GSK-3 is important for recycling and maximal surface expression of Glut1 protein, although precise mechanisms remain to be determined.
RESTING T CELLS
Cytokines and growth factors
T cells exit the thymus and enter the peripheral circulation as quiescent cells with modest energetic and biosynthetic requirements [23]. A key regulatory step for lymphocyte metabolism is control of nutrient uptake. In particular, T cells require extracellular signals, such as cytokines or growth factors, including IL-2, IL-4, and IL-7 or low-level TCR stimulation to maintain glucose uptake. In the absence of extrinsic signals, naïve T cells internalize Glut1 and other nutrient transporters, thus preventing adequate uptake of extracellular nutrients to maintain viability [10, 15, 30, 32,33,34,35]. Conversely, addition of excess growth factor or cytokine stimulation can increase the surface Glut1 and glucose metabolism [10].
IL-7 is a prosurvival factor for early thymocytes and for peripheral naïve and memory T cells [36]. In addition, IL-7 may play a key role in regulation of glucose uptake and metabolism in developing and resting T cells [32, 34, 36,37,38,39]. Some cases of SCID in humans are caused by defects in the IL-7R, the common γ-chain (γc; shared with receptors for several other ILs), or the downstream Jak3 kinase that associates with γc [5, 40], all of which result in failed T cell development. Conversely, overexpression of IL-7 or IL-7Rα results in lymphoma or autoimmunity in some tissues [41, 42].
The signaling mechanism by which IL-7 may promote glucose uptake in T cells is beginning to be elucidated, and STAT5 and Akt activation play prominent roles. Full IL-7 signaling depends on phosphorylation of residue Tyr449 on the IL-7Rα, which has been shown to be required for IL-7R to activate the Jak/STAT5 and PI-3K/Akt pathways [43,44,45]. Tyr449 and STAT5 transcription factors are required for normal lymphocyte development, and Tyr449 is required for IL-7 regulation of glucose uptake and surface Glut1 localization [34]. Interestingly, IL-7R signaling in resting T cells results in rapid activation of STAT5 transcriptional activity but delayed yet sustained activation of Akt [34]. Akt and STAT5 appear to be necessary for IL-7 to promote glucose uptake in resting cells. Akt1 deficiency, pharmacological inhibition of PI-3K with LY294002, or depletion of STAT5 by small hairpin RNA led to defective glucose uptake in response to IL-7 [34]. Although STAT5 has not been described to play a significant role in glucose uptake, Akt kinases are known regulators of glucose uptake and metabolism (Fig. 2) [26, 46]. Constitutively active forms of Akt promote glucose consumption and Glut1 trafficking to the cell surface in a variety of systems [14, 47, 48]. Mechanistically, we have shown recently that immediate activation of STAT5 by the IL-7R leads to a STAT5-dependent transcriptional event that allows IL-7 to activate Akt [34], which then can promote trafficking of Glut1 to the cell surface to promote glucose uptake.
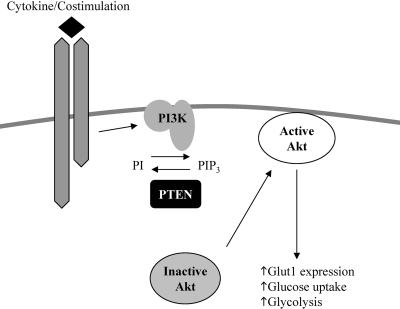
Fig. 2.
Activation of Akt leads to increased glucose uptake. Growth factor or cytokine binding to its receptor on the T cell causes activation of PI-3K, thus converting phosphatidylinositol (PI) to PI 3,4,5 trisphosphate (PIP3), thereby recruiting Akt to the cell surface, where it becomes activated and in turn, up-regulates surface Glut1 expression, glucose uptake, and glycolysis. PTEN, Phosphatase and tensin homologue.
Through this mechanism of delayed, yet chronic, activation of Akt, IL-7 may serve as an ideal T cell survival factor. Unlike many stimulatory cytokines, which lead to an acute activation of Akt, followed by a rapid decline in Akt activity, IL-7 produces a sustained Akt activation to support continued glucose uptake and metabolism. It is now important to understand how STAT5 may alter IL-7R signaling or lead to Akt activation and the role this pathway may play under other circumstances of T cell development and activation or in other tissues in which STAT5 is activated. Other ILs in the class I cytokine superfamily, of which IL-7 is a member, have not been well-studied in regard to their effects on lymphocyte metabolism. However, we have seen that IL-2 and IL-4 may be important in promoting glucose uptake and metabolism in primary T cells [32, 34].
Hormones
In addition to cytokines such as IL-7, immune cells are responsive to hormones and neurotransmitters, some of which may have effects on the metabolism of these cells. A key question for such hormones is to determine if modulation of immune cell function may occur, at least in part, through the regulation of lymphocyte metabolism. One example of such a hormone is insulin. The insulin receptor is not found on resting T cells but is up-regulated significantly on T cells activated by mitogen or antigen [49, 50]. Insulin signaling in T cells causes increased glucose uptake, amino acid transport, lipid metabolism, and protein synthesis and promotes T cell activation and responsiveness [49, 51]. Insulin has also been shown to promote Th2 T cell differentiation [50], which may suppress inflammation. Thus, insulin resistance in obesity and diabetes may enhance Th1 cell development to promote the inflammatory condition observed in the metabolic syndrome.
Another example of a hormone that has important effects on immunity and metabolism is growth hormone. In addition to its well-known effects on growth of cartilage and bone, growth hormone can also affect carbohydrate, lipid, and protein metabolism [52]. Specifically, growth hormone increases protein synthesis by enhancing amino acid uptake and by increasing mRNA transcription and protein translation. Growth hormone causes the direct release of fatty acids from adipose tissue and up-regulates fatty acid oxidation, which also has the result of decreasing protein catabolism. Finally, growth hormone decreases carbohydrate use and impairs glucose uptake into some cells by antagonizing the effects of insulin. Many effects of growth hormone may be mediated by insulin-like growth factors (IGFs) produced largely in the liver following growth hormone signal. In the immune system, growth hormone and IGF-1 have been found to increase the total number of bone marrow B-lineage cells to increase thymic cellularity in rodents and to increase the proliferation of normal and leukemic T cells as well as promote the generation of cytotoxic T lymphocytes [53, 54]. Growth hormone can also stimulate NK cells and granulocyte activity and has been found to be important in reversing steroid-induced leukopenia and inhibition of T cell proliferation [53, 54]. Interestingly, growth hormone is a member of the class I cytokine superfamily, and its signaling relies in large part on STAT5 activation, which we have shown to be required for IL-7 signaling of Glut1 surface expression. To our knowledge, it has yet to be studied whether the role of growth hormone in immune cell function is in any way mediated by its effects on metabolism.
Adipocytokines (or adipokines) are hormones that are secreted by adipose tissue and have effects on the energy status and immune reactivity of the organism. The growing list of known adipocytokines (adipocyte-derived molecules that bridge metabolism and immune function) includes adiponectin, leptin, IL-1, IL-6, IFN-γ, TNF-α, and certain chemokines. Although adiponectin has recently been shown to be a potent growth factor for hematopoietic stem cells [55], leptin has been most directly implicated as a possible bridge between immune function and metabolic status.
Leptin is an adipokine that decreases food intake, increases energy expenditure, and reduces body weight by acting directly on the hypothalamus, where feeding regulation occurs [56]. In addition to its effects on the hypothalamus, leptin acts directly on T cells, where it enhances the production of Th1-type cells, promoting inflammation [57,58,59,60,61]. Furthermore, leptin has been shown to have direct effects on lymphocyte proliferation [58, 59] and protection from apoptosis [62,63,64]. Mice and humans lacking leptin (ob/ob mice) or its receptor (db/db mice) have defects in cell-mediated and humoral-mediated immunity [65, 66]. Congenital leptin deficiency in humans [67,68,69] is associated with recurrent infections and atopic disease as a result of abnormal T cell number and function, with a decrease in CD4 cells and reduced T cell production [70]. Administration of recombinant human leptin to individuals with congenital deficiencies reverses the immune deficit and leads to a switch from predominantly Th2 cytokine to Th1 cytokine secretion [70].
A key remaining question is to determine how the metabolic and immune modulatory functions of leptin interact. Leptin signals via the Jak/STAT pathway, similar to cytokines known to affect cellular glucose metabolism of T cells, such as IL-2 and IL-7 [57, 63, 71], and as such, may be able to increase Glut1 surface expression and glucose uptake in lymphocytes. This seems likely, as it has been shown to increase glucose metabolism in muscle and fat cells [72]. In addition, leptin has been shown to up-regulate adenosine monophosphate-activated kinase (AMPK) activity in muscle cells and may also do so in lymphocytes [71]. Therefore, leptin appears to be a metabolic regulator at the cellular level in immune cells. It remains to be seen whether the effect of leptin on T cell metabolism mediates or influences the leptin-induced effects on T cell number and function. Understanding the function of leptin in immunity is important, as like insulin, leptin has been implicated as a possible mediator in the metabolic syndrome. Leptin is secreted in proportion to body adiposity or total fat volume. As leptin functions as a proinflammatory cytokine, it may be an important link between obesity and the inflammation seen in the metabolic syndrome.
GLUCOSE METABOLISM IN ACTIVATION OF T CELLS
TCR activation
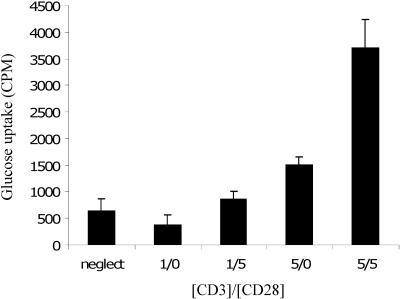
A functional immune response requires rapid and extensive cell growth, proliferation, and production of effector cytokines. As discussed above, the metabolic and biosynthetic demand of lymphocytes becomes dramatically increased after activation. To accommodate this metabolic demand, activated T cells greatly up-regulate Glut1 expression and glucose metabolism [14, 15]. Increased Glut1 and glucose uptake correlate with increased growth and proliferation, and insufficient glucose leads to deficient responses of activated T cells (decreased T cell proliferation and cytokine production) and induction of proapoptotic Bcl-2 family members [26]. The full activation of T cells requires two signals: TCR signal and costimulation via CD28. Costimulatory signals are sufficient and required for maximal increases in glucose uptake (Fig. 3) [15].
Fig. 3.
Activated T cells take up more glucose. T cells were isolated from wild-type mice and cultured for 24 h without treatment (neglect) or with 1 or 5 μg/ml anti-CD3, with or without 5 μg/ml anti-CD28. Glucose uptake of live cells was determined.
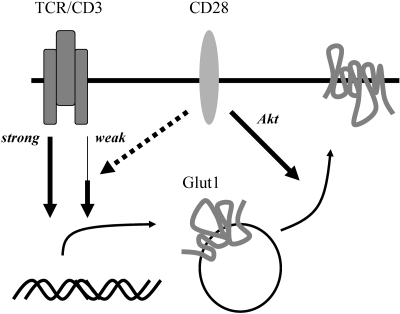
The TCR and costimulatory signal appear to mediate regulation of glucose metabolism through separate pathways that induce Glut1 expression and Glut1 trafficking to the cell surface. Costimulatory signals may help increase Glut1 protein levels under suboptimal TCR stimulation, but they are not required for Glut1 up-regulation with strong antigen receptor stimulation. Despite the ability of strong TCR signals to potently induce Glut1 expression, this does not increase glucose uptake to a level comparable with T cells stimulated with TCR signal accompanied with costimulation (Fig. 4).
Fig. 4.
CD28 costimulation influences Glut1 trafficking. High levels of TCR signal alone are sufficient to increase Glut1 protein levels without costimulation. Low levels of TCR signal require costimulation to increase Glut1 protein levels. CD28 costimulation promotes Glut1 trafficking.
Importantly, we have found that CD28 costimulation is required for efficient trafficking of Glut1 to the cell surface in activated cells [15]. Glut1 localization to the cell surface can be regulated by the PI3K/Akt pathway [30], which is potently activated by the CD28 costimulatory signal [73]. Consistent with this role for Akt, transgenic expression of constitutively active Akt increased T cell size and glucose uptake and decreased dependence on CD28 during TCR stimulation [48]. Constitutively active Akt did not, however, alter Glut1 protein levels and was capable of increasing glucose uptake only if Glut1 protein levels were induced by an independent pathway, such as through strong TCR signaling [15]. Furthermore, transgenic T cells, which overexpress Glut1 and the constitutively active form of Akt, demonstrated increased glucose uptake, cell size, expression of activation markers, and proliferation compared with T cells expressing either single transgene alone [15]. Together, these data support a model in which immune signaling though the TCR-induced signaling pathways is responsible for increased Glut1 protein, and CD28 costimulation via Akt promotes Glut1 trafficking.
AMPK
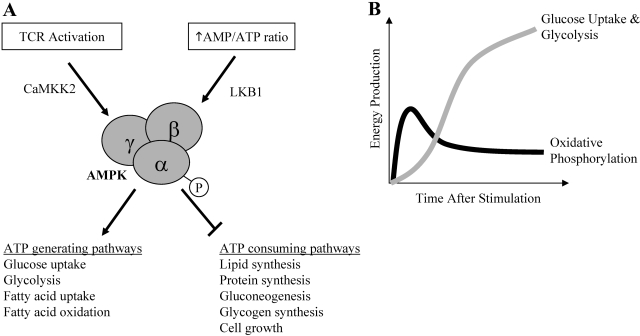
AMPK is also a key regulator of cellular metabolism, which may play a role in the bioenergetics of activated T cells. AMPK promotes ATP conservation and production in times of ATP deficiency. Therefore, ATP-producing pathways such as glycolysis and fatty acid oxidation are turned on, and ATP-consuming pathways such as protein synthesis, fatty acid synthesis, gluconeogenesis, and glycogen synthesis are decreased. AMPK may also further increase the intrinsic activity of Glut1 [74,75,76]. AMPK is activated primarily through two pathways. In times of energetic stress, AMPK is activated by an increased AMP:ATP ratio and the AMPK kinase LKB1 a serine/threonine kinase [74, 77]. In addition, Ca2+-calmodulin-dependent kinase kinase 2 (CAMKK2) has been shown to activate AMPK, independent of AMP levels [78,79,80,81] (Fig. 5A).
Fig. 5.
AMPK activation in T cells. (A) AMPK is activated in T cells in one of two ways: by energy deprivation via LKB1 kinase or by TCR activation via CAMKK2. AMPK activation causes an increase in ATP-generating metabolic processes while decreasing ATP-consuming processes. (B) Within minutes of TCR signaling, AMPK activity is up-regulated, and ATP is most likely generated via oxidative phosphorylation. Within a few hours, Glut1 can be trafficked to the cell surface, and new Glut1 protein is expressed such that glucose uptake stimulates aerobic glycolysis.
AMPK is activated early in T cell activation in response to TCR stimulation, despite ample energy stores [81]. Inhibition of CAMKK activity with pharmacologic inhibitor STO-609 prevented TCR-stimulated AMPK activation, whereas inhibition of PI-3K activity by LY294002 had no effect on AMPK activation, demonstrating that the activation of AMPK following TCR stimulation is dependent on CaMKK but not on the PI-3K signal [81]. Thus, AMPK can be activated in T cells following TCR stimulation or energy stress/depletion of ATP via different upstream kinases [76]. The activation of a T cell leading to increased AMPK activity may be an example of a cell anticipating an increase in energy demands and a mechanism to increase cellular energy prior to the elevation of glucose metabolism as the primary energy source (Fig. 5B).
ALTERED GLUCOSE METABOLISM
Glucose uptake is tightly regulated in lymphocytes, and it was unclear what role increased metabolism may have on T cell activation and survival. To investigate the effects of increased glucose uptake on T cell function, our laboratory has created a transgenic mouse to overexpress Glut1 in T cells [15]. The increase in T cell Glut1 expression leads to increased cell size, increased glucose uptake in peripheral T cells, enhanced IL-2 production in cells stimulated at the TCR (alone or with CD28 costimulation), and enhanced IFN-γ production in cells stimulated with TCR alone [15]. Glut1 overexpression further enhanced activation marker (CD25, CD44, and CD69) expression in T cells, as measured by FACS [15]. Ultimately, transgenic animals developed hyperimmunoglobulinemia and immune complex deposition in the kidneys. Together, these data show that increased Glut1 expression and glucose uptake augment T cell growth and cytokine secretion upon stimulation. Thus, glucose uptake is a limiting factor for T cell growth and stimulation and must be regulated to prevent immune pathology.
Increased glucose uptake may lead to excessive T cell activity and accumulation as a result of enhanced T cell activation and/or inhibition of T cell death following stimulation. Our laboratory has identified a novel, glucose-initiated signaling pathway that leads to inhibition of GSK-3 [82]. This pathway is initiated by alterations in intracellular lipid synthesis of glycolytic cells and activation of protein kinase Cs (PKCs), which then phosphorylate to inactivate GSK-3. Inhibition of GSK-3 will augment T cell signaling through increased nuclear transport of the T cell activation transcription factor NF-AT [83] and prevent cell death through stabilization of the antiapoptotic Bcl-2 family protein, Mcl-1 [82]. Normally, Mcl-1 is targeted for proteasomal degradation by the ubiquitin E3 ligases, Mule and β-transducin repeat-containing protein, after phosphorylation by GSK-3 [84, 85]. Mcl-1, in highly glycolytic cells with inhibition of GSK-3, however, remains unphosphorylated and is not degraded. This stabilization of Mcl-1 increases the threshold for cell death, thus maintaining cell survival. This pathway is one of the first demonstrations of a direct link between glucose metabolism and apoptotic regulatory machinery and will be important for future study to better define the mechanisms of PKC activation, as well as to understand how alterations in GSK-3 activity affect T cell activation and survival.
These findings may be relevant to the fields of cancer biology and diabetes. Many cancer cells show increased levels of glucose uptake and glycolysis, resulting in increased anaerobic metabolism, as described many years ago by Otto Warburg [86], and now termed the Warburg effect [13]. With emerging evidence for metabolic regulation of apoptosis, including our observations in hematopoietic cells, the Warburg effect appears to contribute to cancer cells’ resistance to apoptosis [82] and may aid in identifying metabolic pathways, which can be targeted by chemotherapeutic agents, such as pyruvate kinase M2 isoform [87] and citrate lyase [88]. In obesity and type II diabetes, hyperglycemia and hyperlipidemia lead to an inflammatory environment that can contribute to the constellation of pathologies known as the metabolic syndrome. It is not entirely clear how altered nutrient levels in the serum may affect lymphocyte function. Given the inhibition of T cell function in low glucose levels (Fig. 1), the high-glucose consumption associated with T cell stimulation (Fig. 3), and the hyper-responsiveness of Glut1 transgenic T cells [15], however, it is tempting to speculate that high glucose and lipid levels may contribute to lymphocyte activity to promote inflammation. The roles of nutrient limitation or excess in immunity are important areas for future study and consideration.
CONCLUSION
T cells require glucose uptake for survival and function. Naïve or quiescent T cells require extrinsic cytokine stimulation to maintain glucose uptake for housekeeping functions; this is mediated by and dependent on activation of STAT5 and PI-3K/Akt activity. Activated T cells require a large increase in energy to grow, proliferate, and perform effector functions required of an activated lymphocyte. Energy production in the activated T cell may initially rely on AMPK activity for maximal ATP production and later, rely on changes in Glut1 expression, glucose uptake, and aerobic glycolysis through TCR-mediated Glut1 induction and costimulation-mediated trafficking of Glut1 to the cell surface. Given this critical dependence on glucose, hypo- or hyperglycemic states are problematic. Without enough energy, activated T cells undergo apoptosis; however, overproduction of immune activation or excessive inflammation can lead to cancer or autoimmunity and may affect longevity. Therefore, fine regulation of the system is required, and understanding these metabolic pathways may lead to therapeutic strategies to target immunodeficiencies, cancer, and autoimmunity.
References
- Morgan T E, Wong A M, Finch C E. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri C R, Caruso M, Colonna-Romano G, Grimaldi M P, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Brod S A. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000;49:561–570. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- Buckley R H. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- Bental M, Deutsch C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn Reson Med. 1993;29:317–326. doi: 10.1002/mrm.1910290307. [DOI] [PubMed] [Google Scholar]
- Fox C J, Hammerman P S, Thompson C B. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Krauss S, Brand M D, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- Frauwirth K A, Thompson C B. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M G, Plas D R, Rathmell J C, Fox C J, Harris M H, Thompson C B. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R G, Thompson C B. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Shaw R J. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Kim J W, Dang C V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- Frauwirth K A, Riley J L, Harris M H, Parry R V, Rathmell J C, Plas D R, Elstrom R L, June C H, Thompson C B. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Jacobs S R, Herman C E, MacIver N J, Wofford J A, Wieman H L, Hammen J J, Rathmell J C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner E F, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994;269:31484–31490. [PubMed] [Google Scholar]
- Proud C G. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- Scheepers A, Joost H G, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–371. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- Wood I S, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- Koistinen H A, Zierath J R. Regulation of glucose transport in human skeletal muscle. Ann Med. 2002;34:410–418. doi: 10.1080/078538902321012351. [DOI] [PubMed] [Google Scholar]
- Dugani C B, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R T, Kanzaki M, Pessin J E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Rathmell J C, Vander Heiden M G, Harris M H, Frauwirth K A, Thompson C B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Macheda M L, Rogers S, Best J D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Manel N, Battini J L, Taylor N, Sitbon M. HTLV-1 tropism and envelope receptor. Oncogene. 2005;24:6016–6025. doi: 10.1038/sj.onc.1208972. [DOI] [PubMed] [Google Scholar]
- Rathmell J C, Fox C J, Plas D R, Hammerman P S, Cinalli R M, Thompson C B. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Katagiri H, Takata K, Lin J L, Ishihara H, Inukai K, Tsukuda K, Kikuchi M, Hirano H, Yazaki Y. The role of N-glycosylation of GLUT1 for glucose transport activity. J Biol Chem. 1991;266:24632–24636. [PubMed] [Google Scholar]
- Lachaal M, Spangler R A, Jung C Y. Adenosine and adenosine triphosphate modulate the substrate binding affinity of glucose transporter GLUT1 in vitro. Biochim Biophys Acta. 2001;1511:123–133. doi: 10.1016/s0005-2736(01)00272-3. [DOI] [PubMed] [Google Scholar]
- Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes C P, Holman G D, Whetton A D, Owen-Lynch P J, Baldwin S A. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem. 2003;278:39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- Wieman H L, Wofford J A, Rathmell J C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S A, Barros L F, Griffiths M. Trafficking of glucose transporters—signals and mechanisms. Biosci Rep. 1995;15:419–426. doi: 10.1007/BF01204346. [DOI] [PubMed] [Google Scholar]
- Rathmell J C, Farkash E A, Gao W, Thompson C B. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Vella A, Teague T K, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford J A, Wieman H L, Jacobs S R, Zhao Y, Rathmell J C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger A L, Cinalli R M, Thompson C B. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev Cell. 2003;5:571–582. doi: 10.1016/s1534-5807(03)00291-0. [DOI] [PubMed] [Google Scholar]
- Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Surh C D. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittipatarin C, Khaled A R. Interlinking interleukin-7. Cytokine. 2007;39:75–83. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034–1042. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- Barata J T, Silva A, Brandao J G, Nadler L M, Cardoso A A, Boussiotis V A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Ziegler S F, Buckley R H, Leonard W J. Defective IL7R expression in T(–)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Rich B E, Campos-Torres J, Tepper R I, Moreadith R W, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177:305–316. doi: 10.1084/jem.177.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit S J, Stephan R P, Kim H G, Herrin B R, Gartland L, Klug C A. Determination of lymphoid cell fate is dependent on the expression status of the IL-7 receptor. EMBO J. 2003;22:5511–5521. doi: 10.1093/emboj/cdg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran A E, Smart F M, Cowling R J, Crompton T, Owen M J, Venkitaraman A R. The interleukin-7 receptor α chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 1996;15:1924–1932. [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Li W Q, Hofmeister R R, Young H A, Hodge D R, Keller J R, Khaled A R, Durum S K. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L C, Dhanji S, Snow J W, Priatel J J, Ma M C, Miners M J, Teh H S, Goldsmith M A, Abraham N. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R α mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrom R L, Bauer D E, Buzzai M, Karnauskas R, Harris M H, Plas D R, Zhuang H, Cinalli R M, Alavi A, Rudin C M, Thompson C B. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Rathmell J C, Elstrom R L, Cinalli R M, Thompson C B. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- Stentz F B, Kitabchi A E. Activated T lymphocytes in Type 2 diabetes: implications from in vitro studies. Curr Drug Targets. 2003;4:493–503. doi: 10.2174/1389450033490966. [DOI] [PubMed] [Google Scholar]
- Viardot A, Grey S T, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148:346–353. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- Helderman J H. Role of insulin in the intermediary metabolism of the activated thymic-derived lymphocyte. J Clin Invest. 1981;67:1636–1642. doi: 10.1172/JCI110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M. Growth hormone and metabolism. Diabetes Metab Rev. 1988;4:391–414. doi: 10.1002/dmr.5610040406. [DOI] [PubMed] [Google Scholar]
- Weigent D A. Immunoregulatory properties of growth hormone and prolactin. Pharmacol Ther. 1996;69:237–257. doi: 10.1016/0163-7258(96)00001-0. [DOI] [PubMed] [Google Scholar]
- van Buul-Offers S C, Kooijman R. The role of growth hormone and insulin-like growth factors in the immune system. Cell Mol Life Sci. 1998;54:1083–1094. doi: 10.1007/s000180050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- Wauters M, Considine R V, Van Gaal L F. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol. 2000;143:293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- Matarese G, Moschos S, Mantzoros C S. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- Lord G M, Matarese G, Howard J K, Baker R J, Bloom S R, Lechler R I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gomez-Reino J, Gualillo O. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006;45:944–950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- Loffreda S, Yang S Q, Lin H Z, Karp C L, Brengman M L, Wang D J, Klein A S, Bulkley G B, Bao C, Noble P W, Lane M D, Diehl A M. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–26. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanassoglou E, El-Haschimi K, Li X C, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- Howard J K, Lord G M, Matarese G, Vendetti S, Ghatei M A, Ritter M A, Lechler R I, Bloom S R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R K. Cell-mediated immunity in genetically obese (C57BL/6J ob/ob) mice. Am J Clin Nutr. 1980;33:13–16. doi: 10.1093/ajcn/33.1.13. [DOI] [PubMed] [Google Scholar]
- Mandel M A, Mahmoud A A. Impairment of cell-mediated immunity in mutation diabetic mice (db/db) J Immunol. 1978;120:1375–1377. [PubMed] [Google Scholar]
- Ozata M, Ozdemir I C, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, Cheetham C H, Earley A R, Barnett A H, Prins J B, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg A D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Farooqi I S, Matarese G, Lord G M, Keogh J M, Lawrence E, Agwu C, Sanna V, Jebb S A, Perna F, Fontana S, Lechler R I, DePaoli A M, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Bates S H, Gardiner J V, Jones R B, Bloom S R, Bailey C J. Acute stimulation of glucose uptake by leptin in l6 muscle cells. Horm Metab Res. 2002;34:111–115. doi: 10.1055/s-2002-23192. [DOI] [PubMed] [Google Scholar]
- Parry R V, Reif K, Smith G, Sansom D M, Hemmings B A, Ward S G. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- Hardie D G, Hawley S A, Scott J W. AMP-activated protein kinase—development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G A, Da Silva Xavier G, Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375:1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K, Ingram J C, Porras O H, Barros L F, Hudson E R, Fryer L G, Foufelle F, Carling D, Hardie D G, Baldwin S A. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Hardie D G. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hurley R L, Anderson K A, Franzone J M, Kemp B E, Means A R, Witters L A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong S P, Momcilovic M, Johnstone S R, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Hawley S A, Pan D A, Mustard K J, Ross L, Bain J, Edelman A M, Frenguelli B G, Hardie D G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Tamas P, Hawley S A, Clarke R G, Mustard K J, Green K, Hardie D G, Cantrell D A. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Altman B J, Coloff J L, Herman C E, Jacobs S R, Wieman H L, Wofford J A, Dimascio L N, Ilkayeva O, Kelekar A, Reya T, Rathmell J C. Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ding Q, He X, Hsu J M, Xia W, Chen C T, Li L Y, Lee D F, Liu J C, Zhong Q, Wang X, Hung M C. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Christofk H R, Vander Heiden M G, Harris M H, Ramanathan A, Gerszten R E, Wei R, Fleming M D, Schreiber S L, Cantley L C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer D E, Andreadis C, Shaw A N, Dhanak D, Hingorani S R, Tuveson D A, Thompson C B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]