Abstract
High-throughput screening (HTS) assays used in drug discovery frequently use reporter enzymes such as firefly luciferase (FLuc) as indicators of target activity. An important caveat to consider, however, is that compounds can directly affect the reporter, leading to nonspecific but highly reproducible assay signal modulation. In rare cases, this activity appears counterintuitive; for example, some FLuc inhibitors, acting through posttranslational Fluc reporter stabilization, appear to activate gene expression. Previous efforts to characterize molecules that influence luciferase activity identified a subset of 3,5-diaryl-oxadiazole-containing compounds as FLuc inhibitors. Here, we evaluate a number of compounds with this structural motif for activity against FLuc. One such compound is PTC124 {3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid}, a molecule originally identified in a cell-based FLuc assay as having nonsense codon suppression activity [Welch EM, et al., Nature (2007) 447:87–91]. We find that the potency of FLuc inhibition for the tested compounds strictly correlates with their activity in a FLuc reporter cell-based nonsense codon assay, with PTC124 emerging as the most potent FLuc inhibitor (IC50 = 7 ± 1 nM). However, these compounds, including PTC124, fail to show nonsense codon suppression activity when Renilla reniformis luciferase (RLuc) is used as a reporter and are inactive against the RLuc enzyme. This suggests that the initial discovery of PTC124 may have been biased by its direct effect on the FLuc reporter, implicating firefly luciferase as a molecular target of PTC124. Our results demonstrate the value of understanding potential interactions between reporter enzymes and chemical compounds and emphasize the importance of implementing the appropriate control assays before interpreting HTS results.
Keywords: reporter gene assays, high-throughput screening, Ataluren
Candidate molecules for drug development are increasingly derived from high-throughput screening (HTS) of compound libraries (1). Reporter enzymes such as firefly luciferase (FLuc) are commonly developed to monitor the effects of chemical compounds on target or pathway activity in HTS (2, 3). However, off-target activity such as assay signal modulation that occurs because of a direct interaction of a compound with the reporter enzyme can lead to the false assumption that the compound is active against the target. One such example of off-target activity occurs when a compound directly binds to, and stabilizes, the reporter protein (4).
Reporter enzymes (E), specifically FLuc, can be stabilized by inhibitors (I) if the E·I complex formed is more resistant to degradation than free, unliganded enzyme (4, 5). Firefly luciferase is an exquisitely sensitive reporter (2) with a half-life of ≈4 h; however, even a modest increase in half-life caused by formation of an E·I complex within the cells can lead to significant increases in reporter levels at typical reporter gene assay incubation times (4, 6, 7). Although a stable E·I complex is formed within the cell, detection of reporter enzyme activity typically involves the addition of excess reporter substrates, which can effectively compete off stabilizing inhibitors [Fig. 1 and see supporting information (SI) Appendix, Figs. S1–S3]. Therefore, reporter enzyme activity is detected readily despite the prior formation of an E·I complex within the cellular milieu. In fact, we have found that because of the accumulation of stabilized reporter enzyme, apparent enhancement of reporter activity is often observed (4).
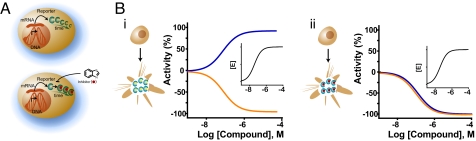
Fig. 1.
Posttranslational reporter stabilization in gene activation assays. (A) Luciferase reporter protein half-life differs in cells not treated (Upper) and treated (Lower) with an inhibitor of the luciferase enzyme. (Lower) Interaction of the inhibitor with the luciferase protein stabilizes the protein, thereby increasing the protein half-life and allowing increased accumulation of reporter protein in the cell. (B) Level of apparent reporter activation depends on properties of the luciferase inhibitor and the assay detection protocol used. After incubation with compound, the cells are lysed, and luciferase detection reagents are added, which may result in displacement of the inhibitor from the reporter (i, illustrated in the schematic of cells) or the inhibitor may remain bound to the reporter (ii, illustrated in the schematic of cells). Shown is the theoretically observed activity of the reporter in the cell-based assay (blue lines) and activity against the reporter enzyme determined in a biochemical assay where substrates are at low concentrations (e.g., ≤Km; orange lines). (Insets) Increase in enzyme concentration in the cell-based assay as a result of inhibitor-based stabilization. (i) If the cells are treated with a compound that acts as a reversible competitive inhibitor of luciferase (orange curve), after interaction and stabilization of the luciferase protein (Inset), the compound can be effectively competed off the luciferase enzyme after cell lysis and treatment with detection reagent containing excess substrates. In this scenario, apparent activation is observed in the cell-based assay (blue curve) that parallels the inhibition of the reporter enzyme in a cell-free, biochemical assay (orange curve). Alternatively, reversible noncompetitive inhibitors may be removed by washing the cells free of compound before detection resulting in the same apparent activation. (ii) If the cells are treated and detected in the presence of a compound that acts as an irreversible or a noncompetitive inhibitor of the luciferase enzyme (orange curve) where the inhibitor is not removed before detection, stabilization of the reporter enzyme still occurs (Inset), but in this scenario inhibition is likely observed (blue curve) because the inhibitor is not displaced from the enzyme during detection. More complex apparent activation behavior such as bell-shaped concentration–response curves may also occur, which are further detailed in SI Appendix.
We have profiled the Molecular Libraries Small Molecule Repository (MLSMR), a collection of structurally diverse compounds representative of those tested in drug discovery HTS, using a FLuc biochemical assay (see PubChem; http:/pubchem.ncbi.nlm.nih.gov). In this effort, we determined the IC50 values for 70,000 samples from the MLSMR and identified several classes of FLuc inhibitors that included 71 compounds with a 3,5-diaryl-oxadiazole core (8); (see also SI Appendix, Table S1). Subsequent work demonstrated that 3,5-diaryl-oxadiazoles appeared to cause an activation phenotype in cell-based reporter gene assays employing expression of wild-type FLuc (SI Appendix, Fig. S3) caused by interaction and stabilization of the compound with the luciferase enzyme (4).
A 3,5-diaryl-oxadiazole that has received significant attention because of its purported activity as a nonsense codon suppressor is {3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid} (PTC124) (Fig. 2). This compound was originally identified through HTS for small molecules that increased the frequency of readthrough of a premature nonsense mutation in a FLuc gene (9). The cell-based assay used to test compounds for this activity relied on the detection of increased FLuc activity, caused, presumably, by increased translation of full-length FLuc protein as a result of nonsense codon suppression activity by the small molecule. However, we noted that the 3,5-diaryl-oxadiazole scaffold is associated with inhibition of FLuc (8). In the initial characterization of PTC124, FLuc activity was shown to increase whereas FLuc mRNA levels were unchanged in the presence of PTC124 (9); though consistent with these data, the possibility of reporter stabilization was not investigated. Therefore, we were interested in examining whether the activity of PTC124 and other 3,5-diaryl-oxadiazole analogs could be attributed to posttranslational stabilization of the FLuc reporter itself.
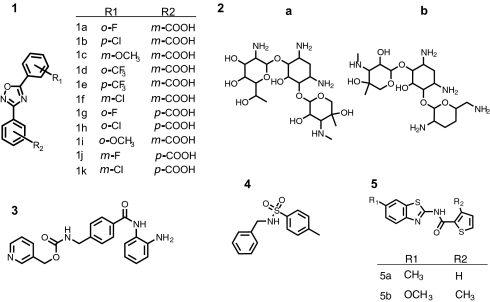
Fig. 2.
Structures of compounds used to evaluate the assays. Shown are the 3,5-diaryl-oxadiazole core (1) present in PTC124 (1a) and analogs synthesized in this work (1 b–k), aminoglycosides G418 (2a) and gentamicin (2b), the HDAC inhibitor MS-275 (3), the RLuc inhibitor 4-methyl-N-(phenylmethyl)benzenesulfonamide (4), and the benzthiazole-based FLuc inhibitors (5 a and b).
There are several testable scenarios that could explain the activity of PTC124 in cell-based assays, including transcriptional activation, posttranscriptional mechanisms such as mRNA stabilization and nonsense codon suppression, and posttranslational stabilization of reporter enzyme levels. In this work we developed luciferase cell-based and enzyme assays to distinguish between these scenarios. We show that although PTC124 and related compounds cause apparent activation in cell-based nonsense codon suppression assays using a FLuc reporter, they are inactive in the same assay when a different bioluminescent reporter, Renilla reniformis luciferase (RLuc) is used as the reporter. Correspondingly, we find that PTC124 is a potent reversible inhibitor of purified FLuc but is inactive against purified RLuc. In fact, we found that the inhibition potency of PTC124 and analogs against purified FLuc matches the potency of activation observed for these compounds in the cell-based nonsense codon suppression assay. Finally, we demonstrate that incubation of purified FLuc with PTC124 protects the protein against degradation by the protease trypsin. Our results therefore indicate that PTC124 interaction with FLuc leading to stabilization of this reporter enzyme is the probable cause for apparent activation of FLuc in cell-based nonsense codon suppression assays.
Results and Discussion
Synthesis of PTC124 and Analogs.
To examine the possibility of a pharmacological connection between the activity of PTC124 in biochemical and cell-based assays involving FLuc, we synthesized PTC124 and 10 analogs (see Fig. 2 and SI Appendix for details on synthesis and characterization). These compounds were used in the experiments described below in an effort to investigate the structure activity relationship in this subclass of 3,5-diaryl-oxadiazoles.
PTC124 Inhibits the FLuc Enzyme and Is Active in a FLuc Nonsense Codon Suppression Cell-Based Assay.
The FLuc cell-based assay was constructed to be similar to that performed by Welch et al. (9) in their discovery of PTC124 (9). We constructed a plasmid containing the coding sequence for FLuc with an in-frame nonsense mutation (UGA) at codon 190 (pFLuc190UGA; SI Appendix, Fig. S4), effectively truncating the polypeptide before the amino acids comprising the substrate-binding pocket. We also developed a purified FLuc enzyme assay to test directly for specific luciferase inhibition.
We evaluated cell-based luciferase reporter activity after transfection of the stop codon construct in the presence of several compound classes (Fig. 2). PTC124 (1a) showed potent inhibition against purified FLuc (Fig. 3Ai, filled circles) and consistent with the original report displayed an activation response in the pFLuc190UGA cell-based assay, here with an EC50 = 30 ± 20 nM (Fig. 3Ai, open circles). The original report stated that maximal activation occurred at ≈3 μM, which is in good agreement with what was observed here given differences in the protocols (e.g., transient vs. stable cell lines).
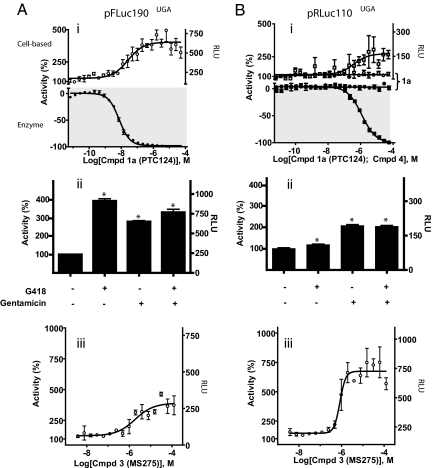
Fig. 3.
PTC124 activity depends on the reporter. (A) (i) (Upper) FLuc activity from Grip-Tite 293 cells transfected with the pFLuc190UGA construct and treated with compound 1a (PTC124) for 24 h (PTC124; open circles) shows concentration-dependent activation. (Lower) Activity of purified FLuc enzyme (with Km concentrations of substrates) shows concentration-dependent inhibition with compound 1a treatment (filled circles). (ii) Aminoglycoside treatment for 24 h with 600 μg/mL G418 or 1 mg/mL gentamicin alone or in combination. Percentage activity of cells with vehicle alone is significantly different from that of cells treated with G418, gentamicin, or both (unpaired t test; *, P < 0.0001 for each comparison; data from 168 assay wells). (iii) FLuc activity from cells transfected with the pFLuc190UGA construct and treated with compound 3, MS-275, for 24 h. (B) (i) RLuc activity is not modulated by compound 1a (PTC124), either in the cell-based assay using Grip-Tite 293 cells transfected with the pRLuc110UGA construct and treated for 72 h with compound (Upper, open circles) or in the biochemical assay using purified RLuc enzyme (with Km concentrations of coelenterazine substrate; Lower, filled circles). Compound 4 shows activation of RLuc in the cell-based assay (Upper, open squares) after 72-h treatment with compound, and inhibition of purified RLuc enzyme (Lower, filled squares). (ii) Antibiotic treatment for 72 h with G418 or gentamicin alone or in combination. Similar to what is seen for pFLuc190UGA experiments, percentage activity for cells without antibiotic treatment is significantly different from that of cells treated with G418, gentamicin, or both (unpaired t test; *, P < 0.0001 for each comparison; data from 168 assay wells). (iii) RLuc activity from cells transfected with the pRLuc110UGA construct and treated with compound 3 for 72 h. Data from duplicate or quadruplicate determinations from experiments performed on separate days (n = 2 or 3) are expressed as the percentage activity ± SEM.
The Cell-Based Nonsense Codon Suppression Assay Is Sensitive to Aminoglycosides and a Histone Deacetylase (HDAC) Inhibitor.
Although we were able to establish that PTC124 caused apparent activation in our cell-based nonsense codon suppression assay, it was important to confirm the sensitivity of our assay to known nonsense codon suppressors: the aminoglycosides G418 and gentamicin. Aminoglycosides are commonly used antibiotics that target and interfere with prokaryotic translation, but they also target eukaryotic 16S rRNA at low affinities (10–13), causing a decrease in fidelity during polypeptide elongation and thus increasing the frequency of reading through a premature termination codon (14).
We found that our pFLuc190UGA cell-based assay was responsive to the aminoglycosides G418 and gentamicin (Fig. 3Aii, compounds 2a and 2b) as has been observed for other cell-based luciferase reporter systems where aminoglycosides can induce nonsense codon suppression in mammalian cells (15–22). Further, we observed that aminoglycoside treatment has the potential to desensitize the assay for nonsense codon suppression activity (Fig. 3Aii). This may explain the lack of response to gentamicin by the FLuc reporter assay described by Welch et al. (9). In this case, maintenance of cell lines that stably express the FLuc reporter may require persistent application of antibiotics, which are commonly aminoglycosides. Our results indicate that this may attenuate any potential assay response to compound-mediated readthrough. For this reason we developed a transient FLuc reporter expression system, which allowed us to omit the antibiotics typically used in selectable marker maintenance (such as G418 or hygromycin B). However, consistent with genuine stop codon suppression, neither compound G418 nor gentamicin inhibited FLuc enzymatic activity (no inhibition at 1–2 mM; SI Appendix, Fig. S6). We also found that our assay system was robustly responsive to the well-used HDAC inhibitor MS-275 (compound 3, Fig. 3Aiii), which significantly increased FLuc activity, presumably via transcriptional activation (23). MS-275 was also determined to be inactive as a direct FLuc inhibitor up to a 125 μM testing concentration (SI Appendix, Fig. S7).
PTC124 Is Specific for FLuc.
We also developed an analogous cell-based assay by using the identical plasmid backbone to pFLuc190UGA, but in this case, replacing the FLuc coding sequence with that of the RLuc coding sequence containing an in-frame stop codon mutation (UGA) at position 110 (pRLuc110UGA; SI Appendix, Fig. S5). This location was chosen because it is not significantly involved in luciferase activity and has been used to create a split RLuc reporter assay where neither enzyme fragments are active alone (24, 25). Therefore, we reasoned that misincorporation of an amino acid at this position because of stop codon suppression, if it occurred, would not adversely affect RLuc enzyme activity.
We next tested compound activity in this identical cell-based nonsense codon assay system by using the unrelated bioluminescent RLuc reporter. Consistent with the lack of PTC124 activity against purified RLuc enzyme (Fig. 3Bi, filled circles), no cell-based activation indicative of apparent readthrough was observed by using the bioluminescent reporter pRLuc110UGA for a 72-h incubation time (Fig. 3Bi, open circles), an incubation time where strong cell-based activation was observed for PTC124 within the FLuc assay (1,300 ± 150% activation; SI Appendix, Fig. S8). If PTC124 was indeed acting via nonsense codon suppression, we would have expected to observe cell-based activation in the pRLuc110UGA assay. We did find, however, that the pRLuc110UGA was responsive to the aminoglycosides G418 and gentamicin (Fig. 3Bii) and to treatment with MS-275 (Fig. 3Biii), suggesting that the transcriptional and translational modulation of the pRLuc110UGA reporter is detectable. Because both reporter constructs contained the same promoter and plasmid backbone, we infer that the PTC124-mediated activity observed only for pFLuc190UGA is not mediated by transcriptional or translational mechanisms.
An RLuc-Specific Inhibitor Shows Apparent Activation in the RLuc Nonsense Codon Suppression Cell-Based Assay.
The selectivity of PTC124 for FLuc and not RLuc is in agreement with determined specificity of FLuc inhibitors containing a 3,5-diaryl-oxadiazole core, determined to be inactive at RLuc (8). We have identified an inhibitor specific for RLuc (compound 4, Fig. 2) with an IC50 = 1.2 ± 0.1 μM (Fig. 3Bi, filled squares). This compound, in fact, was capable of inducing apparent readthrough activity in the cell-based pRLuc110UGA reporter assay (Fig. 3Bi, open squares) with an EC50 = 0.4 ± 0.2 μM. These findings support the existence of a more general phenomenon in which the interaction of a chemical inhibitor with a specific bioluminescent reporter can be the basis of observed apparent readthrough activity in these cell-based assays, without the compound having genuine activity on targets in the intentional biological system.
PTC124 and Analogs Show a Strong Correlation Between FLuc Inhibition and Apparent Activation in the FLuc Nonsense Codon Cell-Based Assay.
We next determined the potencies of PTC124 (1a) and 10 analogs (1 b–k), as well as structurally dissimilar FLuc inhibitors (5 a and b, respectively; Fig. 2), in both the purified FLuc and cell-based pFLuc190UGA assays (Fig. 4 A and B). Comparison of the IC50 of these compounds against purified FLuc with the EC50 for activation in the pFLuc190UGA cell-based assay revealed a strong correlation (r2 = 0.85; Fig. 4C). We noted that the PTC124 analog (1h) that demonstrated >100-fold weaker potency against the FLuc enzyme (IC50 = 1.4 ± 0.7 μM; Fig. 4A, filled squares) also showed a correspondingly weaker activation response in the pFLuc190UGA cell-based assay (EC50 = 0.50 ± 0.14 μM; Fig. 4A, open squares). Additionally, compound 1k, a weak inhibitor against the firefly luciferase enzyme (IC50 = 30 ± 20 μM; Fig. 4A, filled triangles), also demonstrated poor activation in the pFLuc190UGA cell-based assay (EC50 >50 μM; Fig. 4A, open triangles). This trend held for the structurally dissimilar benzthiazole-based firefly luciferase inhibitors, compounds 5a and 5b (Fig. 4B), and additional 3,5-diaryl-oxadiazole analogs (1 b–k), which were found to recapitulate the apparent nonsense codon suppression in pFLuc190UGA assays via “activation” of FLuc activity in the cell-based assay (SI Appendix, Fig. S7). Further, we found that all of these analogs were inactive in the analogous RLuc reporter assay (SI Appendix, Fig. S7). This remarkable pharmacological relationship between FLuc inhibition and cell-based activation activity strongly suggests that inhibition of FLuc and subsequent stabilization of FLuc protein mediate the apparent nonsense codon suppression activity of PTC124.
Fig. 4.
Relationship of PTC124 cell-based activity with FLuc inhibition potency. (A) (Upper) FLuc activity from Grip-Tite 293 cells transfected with the pFLuc190UGA construct and treated with PTC124 (1a, open circles) and PTC124-analogs 1h (open squares) and 1k (open triangles). (Lower) Activity of purified FLuc enzyme with Km concentrations of substrates with treatment of compound 1a (filled circles), 1h (filled squares), and 1k (filled triangles). (B) Activity of benzthiazole-based FLuc inhibitors (5a, circles; 5b, squares) in the cell-based assay with 293 cells transfected with the pFLuc190UGA construct (Upper) and in the purified FLuc assay (Lower). (C) Pharmacological correlation of the EC50 for activation in the pFLuc190UGA cell-based assay with the IC50 derived against purified luciferase enzyme (r2 = 0.85; dashed lines represent the 95% confidence intervals). Mean ± SEM are from two to four replicates that were repeated on 2 separate days.
PTC124 Decreases FLuc Proteolysis, but Not RLuc.
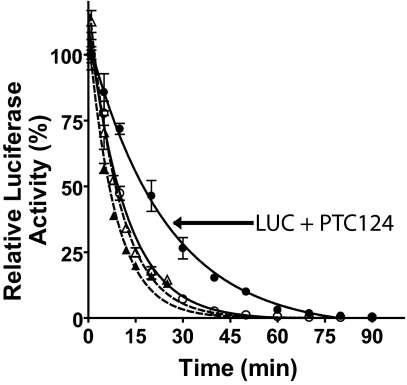
Firefly luciferase contains two domains, linked by a hinge region that, upon substrate binding, reorientate to form a closed conformation (26, 27). Ligand binding to firefly luciferase has been shown to protect the enzyme from proteolysis, and a similar stabilization mechanism has been found for other enzymes (6, 28). We examined the ability of PTC124 to protect purified FLuc and RLuc from trypsin digestion (Fig. 5). We found that 2 μM PTC124 slowed the proteolysis half-life of firefly luciferase by ≈2-fold but showed no effect on the proteolysis half-life of RLuc. This finding supports the premise that an enzyme conformation that is less susceptible to proteolysis is induced in the presence of a potent inhibitor, thereby prolonging half-life of inhibitor-bound FLuc in cells or cell extracts.
Fig. 5.
PTC124 selectively decreases proteolysis of FLuc by trypsin. Purified luciferase enzymes were incubated with trypsin in the presence (filled symbols) and absence (open symbols) of 2 μM PTC124. Aliquots were removed and diluted (90-fold) with the appropriate luciferase detection buffer. Data shown are the mean ± SE from three determinations. Circles, Fluc; triangles, RLuc. Values for t1/2 are: RLuc, 4 ± 0.1 min; RLuc with PTC124, 5 ± 0.2 min; Luc, 8 ± 0.4 min; Luc with PTC124, 17 ± 0.9 min.
Concluding Remarks.
The strong potency of the 3,5-diaryl-oxadiazole PTC124 against FLuc was likely achieved as a result of chemical optimization efforts in which thousands of analogs were prepared based on their activity in the original FLuc cell-based assay performed by Welch et al. (9). Our identification of the 3,5-diaryl-oxadiazole class of FLuc inhibitors emerged from screening the MLSMR (8). The most potent 3,5-diaryl-oxadiazoles identified from the MLSMR screen showed an IC50 ≈0.2 μM, but none of these compounds were subjected to chemical optimization efforts aimed at developing more potent FLuc inhibitors. The >20-fold greater potency of PTC124 was likely caused by the medicinal chemistry efforts aimed at optimizing the apparent readthrough activity as monitored by FLuc reporter activity, mistakenly used as a unique measure of nonsense codon suppression.
We have thus presented four lines of evidence supporting the contention that the initial discovery of PTC124 may be attributable to posttranslational inhibitor-based reporter stabilization. (i) PTC124, a member of the 3,5-diaryl-oxadiazole class of luciferase inhibitors, potently inhibits Fluc. (ii) Structurally unrelated FLuc inhibitors show similar levels of activation in FLuc-dependent reporter assays of nonsense codon suppression. (iii) The cell-based activation of FLuc activity is pharmacologically equivalent to the potency of the compound against the enzymatic reporter. (iv) No apparent nonsense codon suppression is observed when employing a luminescent reporter (RLuc) whose enzymatic activity is refractory to inhibition by PTC124.
Our data show that PTC124 and its analogs mediate activation of FLuc activity in cell-based assays in a manner that is strongly associated with reporter inhibition. Unlike our cell-based nonsense codon suppression assay, which has demonstrated responsiveness to aminoglycosides, known nonsense codon suppressors, the assay described by Welch et al. (9) was unresponsive to tested concentrations of aminoglycosides, suggesting that their assay may have had reduced sensitivity to compound-mediated readthrough. In addition, the cause of increased luciferase activity detected in their assay could be the result of several different mechanisms, none of which could be distinguished: transcriptional activation, nonsense codon suppression, or inhibitor-based reporter stabilization. We demonstrate here that all of these mechanisms can produce activation of the reporter response and that these mechanisms can only be distinguished by implementing analogous alternative reporter assays that serve as appropriate controls. Oftentimes such “counterscreens” can be useful for identifying off-target activity (29, 30). Further, the mechanism of inhibitor-based reporter stabilization is consistent with the production of FLuc from synthetic FLuc mRNA containing different premature termination codons when incubated with HeLa cell-free extracts, as well as the negative data presented in the original study, where it was demonstrated that PTC124 does not produce readthrough of normal or contiguous stop codons or affect mRNA levels (9). Currently, it has been speculated that PTC124 interacts with the ribosome that, through a currently unknown mechanism (31, 32), facilitates readthrough specifically of premature stop codons. However, the simplest explanation for PTC124 activity and the congener structure–activity relationship in the cell-based nonsense codon suppression assay is posttranslational stabilization of the luciferase reporter enzyme.
After the initial identification of PTC124 in the cell-based nonsense codon suppression assays, Welch and coworkers (9, 33) provided evidence of increased full-length protein expression from disease genes containing premature stop codons upon treatment with PTC124. Functional studies have also been performed to show partial rescue of protein function in mouse models and in clinical trials with PTC124 treatment (9, 33, 34). It is thus possible that PTC124 does have nonsense codon suppression activity, in addition to activity as a FLuc inhibitor. However, it is presently not clear how strong activity associated with FLuc inhibitor-based reporter stabilization could have been distinguished from genuine nonsense codon suppression activity because all of the initial assays leading to the discovery of PTC124 were FLuc-based. Therefore, we suggest that the selection of PTC124 as the optimal read through compound be interpreted with this additional mechanism for its activity in mind.
Methods
Luciferase Reporter Assays.
Constructs containing the coding region for wild-type or mutant firefly or RLuc were prepared by GenScript. Site-directed mutagenesis to create premature termination codons within the luciferase genes and subsequent sequencing of the full-length cDNAs were also performed by GenScript. For sequences of each mutagenized construct, see SI Appendix. Purified DNA (10 μg per T175 flask) was transiently transfected into either HEK293 cells or Grip-Tite 293 MSR Cells. Grip-Tite 293 cells are derived from the 293 line of embryonal human kidney cells (Invitrogen) and demonstrate greater adherence. All cell-based assays were performed in 1,536-well plates with an assay volume of 4.5 μL per well. Comparative cell-based experiments were performed with cells from the same batch of transfected cells to control for transfection efficiency. Cells were incubated with compound or antibiotic for 16–72 h. In experiments with Grip-Tite cells, cells were washed with PBS before the addition of detection reagent containing the FLuc or RLuc substrate. Luminescence from luciferase activity was detected by using ViewLux (PerkinElmer). Experimental plates contained the transcriptional activator MS-275 as a positive control and DMSO-treated cells or mock-transfected cells as a negative control. Percentage activity was defined as the percentage signal above DMSO-treated cells. Detailed protocols for the transient transfection and cell-based assays are provided in SI Appendix. Experiments demonstrating inhibition of purified enzyme were conducted in a total assay volume of 4 μL per well in 1,536-well microplates.
Trypsin Digest.
Bovine trypsin was obtained from Sigma as a lyophilized powder and dissolved in 50 mM Tris acetate buffer (pH 7.5) immediately before use (at 20 mg/mL). After mixing of trypsin (170 nM final concentration) with luciferases (450 nM final concentration) in the presence or absence of 2 μM PTC124 in 50 mM Tris acetate (pH 7.5), 10 mM Mg acetate, 0.05% BSA, the reaction was diluted 1:90 with Steady-Glo luciferase (Promega) detection reagent at the appropriate time points, and luminescence was determined on the ViewLux.
Synthesis of PTC124 and Analogs.
The small-molecule PTC124 was prepared by using a method similar to the one described by Welch et al. (9), and synthetic schemes and supporting analytical data are provided in SI Appendix.
Supplementary Material
Acknowledgments.
We thank Paul Shinn for compound management support, Craig Thomas and William Leister for analytical chemistry support, and Noel Southall for data analysis. We thank Christopher Austin and Charles Venditti for critical reading of the manuscript. This work was supported by the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813345106/DCSupplemental.
References
- 1.Bender A, et al. Which aspects of HTS are empirically correlated with downstream success? Curr Opin Drug Discov Devel. 2008;11:327–337. [PubMed] [Google Scholar]
- 2.Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol. 2007;5:127–136. doi: 10.1089/adt.2006.053. [DOI] [PubMed] [Google Scholar]
- 3.Inglese J, et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 4.Auld DS, Thorne N, Nguyen DT, Inglese J. A specific mechanism for nonspecific activation in reporter-gene assays. ACS Chem Biol. 2008;3:463–470. doi: 10.1021/cb8000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson PA, et al. Identification of ligand binding by protein stabilization: Comparison of ATLAS with biophysical and enzymatic methods. Assay Drug Dev Technol. 2008;6:69–81. doi: 10.1089/adt.2007.100. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JF, Hayes LS, Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- 7.Hargrove JL, Schmidt FH. The role of mRNA and protein stability in gene expression. FASEB J. 1989;3:2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- 8.Auld DS, et al. Characterization of chemical libraries for luciferase inhibitory activity. J Med Chem. 2008;51:2372–2386. doi: 10.1021/jm701302v. [DOI] [PubMed] [Google Scholar]
- 9.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 10.Moazed D, Noller HF. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm JM, Pettitt SE, Jessop JJ. Aminoglycoside antibiotics and eukaryotic protein synthesis: Structure–function relationships in the stimulation of misreading with a wheat embryo system. Biochemistry. 1978;17:1143–1149. doi: 10.1021/bi00600a001. [DOI] [PubMed] [Google Scholar]
- 12.Recht MI, Douthwaite S, Puglisi JD. Basis for prokaryotic specificity of action of aminoglycoside antibiotics. EMBO J. 1999;18:3133–3138. doi: 10.1093/emboj/18.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zingman LV, et al. Aminoglycoside-induced translational read-through in disease: Overcoming nonsense mutations by pharmacogenetic therapy. Clin Pharmacol Ther. 2007;81:99–103. doi: 10.1038/sj.clpt.6100012. [DOI] [PubMed] [Google Scholar]
- 14.Hainrichson M, Nudelman I, Baasov T. Designer aminoglycosides: The race to develop improved antibiotics and compounds for the treatment of human genetic diseases. Org Biomol Chem. 2008;6:227–239. doi: 10.1039/b712690p. [DOI] [PubMed] [Google Scholar]
- 15.Cassan M, Rousset JP. UAG readthrough in mammalian cells: Effect of upstream and downstream stop codon contexts reveal different signals. BMC Mol Biol. 2001;2:3. doi: 10.1186/1471-2199-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard MT, et al. Readthrough of dystrophin stop codon mutations induced by aminoglycosides. Ann Neurol. 2004;55:422–426. doi: 10.1002/ana.20052. [DOI] [PubMed] [Google Scholar]
- 17.Diop D, Chauvin C, Jean-Jean O. Aminoglycosides and other factors promoting stop codon readthrough in human cells. C R Biol. 2007;330:71–79. doi: 10.1016/j.crvi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Grentzmann G, et al. A dual-luciferase reporter system for studying recoding signals. RNA. 1998;4:479–486. [PMC free article] [PubMed] [Google Scholar]
- 19.Martin R, et al. Aminoglycoside suppression at UAG, UAA and UGA codons in Escherichia coli and human tissue culture cells. Mol Gen Genet. 1989;217:411–418. doi: 10.1007/BF02464911. [DOI] [PubMed] [Google Scholar]
- 20.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2:467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 21.Burke JF, Mogg AE. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G418 and paromomycin. Nucleic Acids Res. 1985;13:6265–6272. doi: 10.1093/nar/13.17.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuvakhova M, Keeling K, Bedwell DM. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA. 2000;6:1044–1055. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T, et al. Synthesis and histone deacetylase inhibitory activity of new benzamide derivatives. J Med Chem. 1999;42:3001–3003. doi: 10.1021/jm980565u. [DOI] [PubMed] [Google Scholar]
- 24.Loening AM, Fenn TD, Gambhir SS. Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis. J Mol Biol. 2007;374:1017–1028. doi: 10.1016/j.jmb.2007.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefan E, et al. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci USA. 2007;104:16916–16921. doi: 10.1073/pnas.0704257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deluca M. Firefly luciferase. Adv Enzymol Relat Areas Mol Biol. 1976;44:37–68. doi: 10.1002/9780470122891.ch2. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsu T, et al. Structural basis for the spectral difference in luciferase bioluminescence. Nature. 2006;440:372–376. doi: 10.1038/nature04542. [DOI] [PubMed] [Google Scholar]
- 28.Harada N, Honda SI, Hatano O. Aromatase inhibitors and enzyme stability. Endocr Relat Cancer. 1999;6:211–218. doi: 10.1677/erc.0.0060211. [DOI] [PubMed] [Google Scholar]
- 29.Keiser MJ, et al. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor KA, Roth BL. Finding new tricks for old drugs: An efficient route for public-sector drug discovery. Nat Rev Drug Discov. 2005;4:1005–1014. doi: 10.1038/nrd1900. [DOI] [PubMed] [Google Scholar]
- 31.Allamand V, et al. Drug-induced readthrough of premature stop codons leads to the stabilization of laminin α2 chain mRNA in CMD myotubes. J Gene Med. 2008;10:217–224. doi: 10.1002/jgm.1140. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz A, Famulok M. Chemical biology: Ignore the nonsense. Nature. 2007;447:42–43. doi: 10.1038/nature05715. [DOI] [PubMed] [Google Scholar]
- 33.Du M, et al. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerem E, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: A prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.