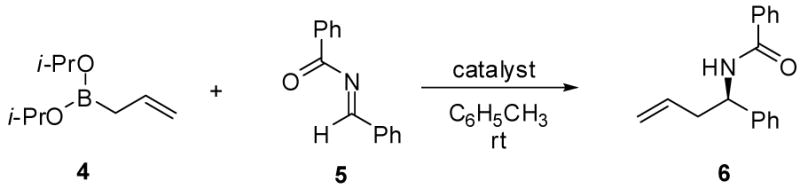
Table 1.
Asymmetric Allylboration of Acyl Iminesa
 | ||||
|---|---|---|---|---|
| entry | catalyst | mol%b | % yieldc | erd |
| 1 | - | - | <5 | - |
| 2 | 7a | 15 | <5 | 50:50 |
| 3 | 7b | 15 | <5 | 55:45 |
| 4 | 7c | 15 | 10 | 60:40 |
| 5 | 7d | 15 | 51 | 50:50 |
| 6 | 7e | 15 | 76 | 68:32 |
| 7 | 7f | 15 | 81 | 93:7 |
| 8 | 7g | 10 | 60 | 96.5:3.5 |
| 9 | 7h | 15 | 85 | 96:4 |
| 10 | 7h | 10 | 80 | 95:5 |
| 11 | 7h | 5 | 60 | 90:10 |
| 12 | 7i | 15 | 21 | 55:45 |
Reactions were run with 0.125 mmol borane, 0.125 mmol acyl imine, 15 mol % catalyst and in toluene (0.1 M) for 16 h under Ar, followed by flash chromatography on silica gel.
Catalyst concentration used relative to imine.
Isolated yield.
Enantiomeric ratios determined by chiral HPLC analysis.
