Table 2.
Asymmetric Allylboration of Acyl Iminesa
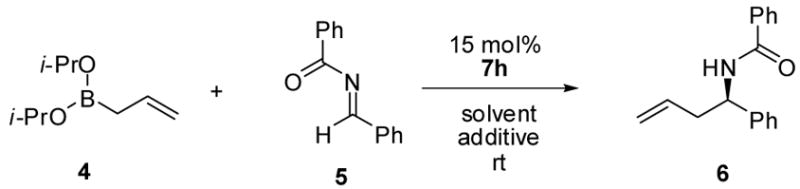 | ||||
|---|---|---|---|---|
| entry | solvent | additive | % yieldb | erc |
| 1 | THF | - | 32 | 58:42 |
| 2 | Et2O | - | 28 | 60:20 |
| 3 | CH2Cl2 | - | 75 | 92:8 |
| 4 | C6H5CH3: C6H5CF3 3:1 | - | 77 | 92:8 |
| 5 | C6H5CH3 | - | 81 | 93:7 |
| 6 | C6H5CH3 | 3Å MS | 87 | 99:1 |
| 7 | C6H5CH3 | 4Å MS | 85 | 97:3 |
| 8 | C6H5CH3 | 5Å MS | 83 | 90:10 |
Reactions were run with 0.125 mmol borane, 0.125 mmol acyl imine, 15 mol % catalyst and in toluene (0.1 M) for 16 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Enantiomeric ratios determined by chiral HPLC analysis.
