Table 3.
Asymmetric Allylboration of Benzoyl Iminesa
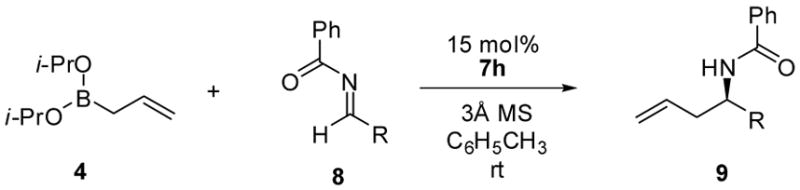 | ||||
|---|---|---|---|---|
| entry | R | product | % yieldb | erc |
| 1 | Ph | 9a | 87 | 99:1 |
| 2 | p-CH3-C6H4 | 9b | 83 | 98:2 |
| 3 | p-Br-C6H4 | 9c | 86 | 97.5:2.5 |
| 4 | p-CH3O-C6H4 | 9d | 85 | 95:5 |
| 5d | p-F-C6H4 | 9e | 94 | 98:2 |
| 6d | o-F-C6H4 | 9f | 91 | 95.5:4.5 |
| 7d | m-CF3-C6H4 | 9g | 89 | 97.5:2.5 |
| 8 | 2-C4H3O | 9h | 83 | 96:4 |
| 9 | 2-C4H3S | 9i | 81 | 95:5 |
| 10 | 2-naphthyl | 9j | 88 | 96:4 |
| 11 | (E)-PhCH=CH | 9k | 82 | 95.5:4.5 |
| 12 | PhCH2CH2 | 9l | 83 | 99.5:0.5 |
| 13 | c-C6H11 | 9m | 80 | 98:2 |
| 14 | t-Bu | 9n | 81 | 99.5:0.5 |
| 15 | BnOCH2 | 9o | 84 | 96.5:3.5 |
| 16 | (Z)-EtCH=CH(CH2)2 | 9p | 82 | 95.5:4.5 |
Reactions were run with 0.5 mmol 4, 0.5 mmol imine, and 15 mol % catalyst and 3Å molecular sieves in toluene for 36 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Determined by chiral HPLC analysis.
Reactions were run at 10 °C for 48 h.
