Abstract
There is a mismatch between the documentation of the visually guided behaviors and visual physiology of decapods (Malacostraca, Crustacea) and knowledge about the neural architecture of their visual systems. The present study provides a description of the neuroanatomical features of the four visual neuropils of the grapsid crab Chasmagnathus granulatus, which is currently used as a model for investigating the neurobiology of learning and memory. Visual memory in Chasmagnathus is thought to be driven from within deep retinotopic neuropil by large field motion sensitive neurons. Here we describe the neural architecture characterizing the Chasmagnathus lobula, in which such neurons are found. It is shown that unlike the equivalent region of insects, the malacostracan lobula is densely packed with columns, the spacing of which is the same as that of retinotopic units of the lamina. The lobula comprises many levels of strata and columnar afferents that supply systems of tangential neurons. Two of these, which are known to respond to movement across the retina, have orthogonally arranged dendritic fields deep in the lobula. They also show evidence of dye coupling. The present results discuss the significance of commonalties across taxa with respect to the organization of the lamina and medulla and contrasts these with possible taxon-specific arrangements of deeper neuropils that support systems of matched filters.
Indexing terms: arthropod, crustacean, insect, vision, learning and memory
INTRODUCTION
In decapod crustaceans, each of the two optic lobes consists of three nested retinotopic neuropils, which with a number of circumscribed protocerebral neuropils are contained in the eyestalk. These neuropils are connected to medial neuropils of the supraesophageal ganglion by discrete protocerebral tracts. From periphery to center the three optic neuropils are traditionally called lamina ganglionaris, external medulla, and internal medulla. However, modern anatomical and developmental studies argue for them being named the lamina, medulla, and lobula, so as to conform with homologous neuropils in insects (Strausfeld and Nässel, 1980; Harzsch, 2002; Sinakevitch et al., 2003).
Compared with insects, relatively little is known about the neural organization and cellular morphologies of crustacean optic neuropils. Studies are restricted to a few species that include entomostracan and malacostracan taxa. Among decapods, the crayfish is the single taxon from which optic lobe neuroanatomy has been studied in most detail (Nässel, 1977; Strausfeld and Nässel, 1980). Similarly, physiological studies of vision in crustaceans are scant. Pioneered by Wiersma and coworkers who extracellularly recorded the response of unidentified neurons from axons in the protocerebral tract (for a review see Wiersma et al., 1982) recent intracellular studies have been principally carried out by Glantz and his colleagues, focusing on neurons of the lamina and medulla (for a review see Glantz and Miller, 2002).
Decapod crustaceans do, however, possess a visual system that is highly accessible for intracellular studies (Berón de Astrada et al., 2001) and, as is abundantly documented, they display elaborated and fascinating visually evoked behaviors. These include navigation and homing, sexual display and aggressive signaling (Land and Layne, 1995; Cannicci et al., 2000; Hemmi and Zeil, 2003; Backwell et al., 2000; Zeil and Hoffmann, 2001) and the formation of social hierarchies (Herberholz et al., 2001; Duffy, 2002). In the crab Chasmagnathus defined visual cues, such as a bar moving at a certain speed across the upper visual field, elicit directional escape responses that have been interpreted as antipredatory (Tomsic et al., 1993). However, these responses are modifiable in that following repetitive presentation of the visual stimulus the response declines and remains reduced for several days. Although at the beginning of our studies with Chasmagnathus we termed this phenomenon habituation (Lozada et al., 1990; Tomsic et al., 1993), later investigations demonstrated that it was a rather more complex form of memory determined by an association between the moving stimulus and the context in which the stimulus occurred (Tomsic et al., 1998; Pedreira et al., 2002, 2004; Pedreira and Maldonado, 2003). This adaptive behavior has not been observed to occur in Pachygrapsus marmoratus, a species close to Chasmagnathus in phylogeny but disparate in ecology. Thus, the ability of Chasmagnathus to modify the response to certain visual stimuli has been interpreted as an adaptation to the conditions of its natural environment (Tomsic et al., 1993; Eckert and Zeil, 2001).
The memory process of Chasmagnathus has been investigated using behavioral, pharmacological, molecular, and electrophysiological approaches (for a review see Maldonado, 2002; Tomsic, 2002). A group of giant visual tangential neurons (called movement detector neurons or MDNs) were identified that respond to the same visual stimuli that lead to the adaptive behavior described above (Berón de Astrada and Tomsic, 2002, Tomsic et al., 2003). MDNs arborize within the lobula and in the lateral protocerebrum, having somata located beneath the lobula. MDNs axons leave the optic lobe through the protocerebral tract. They give rise to numerous branches in the lobula that extend tangentially across retinotopic columns in a manner similar to the orthogonal arrangement of wide-field tangential cells known from studies of dipterous lobula plates (Hengstenberg, 1982; Hausen, 1984). Intracellular recordings also shown that MDNs respond to visual stimuli presented either to the ipsilateral or to the contralateral eye thus demonstrating that processing of binocular visual information is likely to occur at the level of the lobula (Sztarker and Tomsic, 2004). Certain MDNs respond to both visual and mechanical stimuli applied to areas of the body demonstrating that the integration of multimodal information is also likely to occur in the lobula (Berón de Astrada and Tomsic, 2002). A crucial finding has been that MDNs modify their responses during visual learning, whereby the decrement of response by the MDN in response to repeated visual stimulation by a bar to the upper eye field, and the duration of the acquired suppression of the response, correlates strikingly with behavioral results showing adaptation to the same visual stimulation. These studies thus provide the first identification of individual neurons that support long-term memories in an arthropod (Tomsic et al., 2003). The results also challenge the commonly held assumption that optic neuropils of arthropods are sensory centers solely devoted to visual processing. The sum of evidence therefore suggests that the lobula is not simply a visual processing neuropil but that it possesses features that are often ascribed to “higher centers”. It integrates information from both eyes, from different sensory modalities, and from different sensory fields. Further, it supports long-term memory by identified neurons.
If the crustacean lobula has properties of higher brain centers, are such properties suggested by its cellular organization? Might, for example, the crustacean lobula comprise cell assemblies typical of those of identified higher centers, such as the insect mushroom bodies, that are thought to be crucial to the formation of memories (Farris and Sinakevitch, 2003). Is the lobula of crustaceans like that of insects? If so, might visual learning and memory also be supported by the lobulas of this group of arthropods as well? As a first step towards investigating what might be a ubiquitous property of the malacostracan/insect visual system, the present study describes the neuronal organization of the crab optic lobes with emphasis on the arrangements of motion detector neurons in the lobula.
MATERIAL AND METHODS
Animals
Animals were adult male Chasmagnathus granulatus crabs measuring 2.7-3.0 cm across the carapace and weighing about 17 gm (Fig. 1A). Crabs were collected in narrow coastal inlets (rias) of San Clemente del Tuyú, Argentina. Crabs were maintained in the laboratory in plastic tanks (35 × 48 × 27 cm) filled to 2 cm depth with diluted marine water prepared using hw-Marinex (Winex, Hamburg, Germany), having a salinity of 10-14 % and a pH of 7.4-7.6.They were maintained at 22-24°C and on a 12 hours light/dark cycle.
Fig. 1.

The grapsid crab Chasmagnathus granulatus and its visual system. A: An adult male crab with a carapace width of 2.8 cm demonstrating the upright posture of its eyestalks. B: The compound eye and optic neuropils. The right eyestalk, showing the frontal (upper panel) and the lateral (lower panel) appearance of the eye. The diagram to the right of each picture illustrates the retina and the organization and relative dimensions of the optic neuropils inside the eyestalk. Abbreviations: Re, retina; BM, basement membrane; FB, bundles of retinula cell fibers; HS, hemocoelic sinus; La, lamina; Me, medulla; Lo, lobula; LoP, lobula plate; LPc, lateral protocerebrum; PcT, protocerebral tract; D, dorsal; V ventral; L, lateral; M, medial; A, anterior; P, posterior. Scale bar = 500 μm.
Coordinates of the optic lobe and planes of sectioning
Most anatomical studies of crustacean visual system have been performed on species of crayfishes and shrimps, whose eyestalks are oriented almost horizontally. Crab eyestalks are oriented approximately vertical (Fig. 1A). Longitudinal sections are those obtained by performing sections through the optic ganglia along the anteroposterior axis of the animal. Transversal sections are those obtained by sectioning the optic ganglia along the lateromedial axis. Tangential sections are obtained sectioning the optic ganglia tangentially to the surface of the neuropils (Fig. 1B).
Histology and anatomical reconstructions
We used Bodian’s original (1937) reduced-silver method to reveal the neuroarchitecture of the optic lobes of adult crabs. Animals were anesthetized by chilling on ice. Dissected optic lobes were fixed for six hours in AAF (5ml glacial acetic acid, 85 ml ethanol, 10 ml 37% commercial formalin), then washed in 70% ethanol and dehydrated in ascending ethanols. After two immersions in dry 100% ethanol, tissue was cleared in terpineol and then placed in xylol, warmed to 60°C, and then transferred to a 1:1 mixture of Paraplast plus (Corning) and xylol for 20 minutes before two immersions in pure Paraplast each for 30 minutes before hardening. Blocks were serially sectioned at 12-25 μm. Dewaxed sections were incubated overnight at 45°C in 250 ml distilled water containing 1% silver proteinate (Johnson Matthey SA) and 2-7 gm clean copper shot per 100 ml solution. After incubation, tissue was placed in developer, gold toned, reduced and then fixed according to Bodian’s original method. After dehydration, sections were mounted in Entellan under coverslips.
Images from reduced-silver preparations were captured with a Sony DKC 5000 CCD digital camera linked to an Apple G4 computer equipped with graphic software. For each image, between 4 and 16 optical images were captured and layered in register. Images were open in Adobe Photoshop 6.0 and made transparent using the darkening function and then flattened.
MDNs were intracellularly stained with Neurobiotin and then secondarily labeled with rhodamine avidin D (Vector Labs. Inc.) and viewed with an Olympus confocal laser-scanning microscope.
RESULTS
General description of the crab visual system
The eyes of Chasmagnathus are mounted on top of movable stalks that extend approximately vertically above the carapace (Fig. 1A). Each eye consists of about 7,000 ommatidia, which are distributed around the tip of the eyestalk (Fig. 1B) except for a narrow area of cuticle located towards the medial side of the animal. Thus, the visual field of each eye subtends almost the entire panorama surrounding the animal.
The arrangement of optic neuropils within the eyestalk of Chasmagnathus is schematized in figure 1B, and shown in figure 2. Neuropils of the lateral protocerebrum starkly contrast with the optic neuropils whose principal architectural elements are retinotopic columns intersected by layers. Each cartridge represents a visual sampling unit of the retina: eight photoreceptors from each ommatidium send their axons as a coherent bundle (Figs. 3A,B) into a lamina optic cartridge (Fig. 3C), each of which is delineated by a rectilinear organization of tangentially directed processes (Fig. 4). Efferent neurons extending from optic cartridges project the distal representation of visual sampling units proximally into the medulla. Deeper retinotopic organization is preserved through the medulla by its columnar neurons that then map this geometry into the lobula (Fig. 2A).
Fig. 2.
Overview of Chasmagnathus optic lobe stained with Bodian’s reduced-silver. A: Longitudinal section of the entire lobe. Of the three visual neuropils, the medulla (Me) and lobula (Lo) are divided into many discrete strata whereas the lateral protocerebrum (LPc) is organized into discrete neuropil islets or nuclei, connected by tracts. The lamina (La) is connected to the medulla by the first optic chiasma (Och1), and the medulla is connected to the lobula by the second chiasma (Och2). The lobula provides many discrete tracts to the protocerebrum. The neuron somata are arranged as layers or clusters outside neuropils. B: Transversal section of the lobula and lobula plate (LoP), the latter is a small tectum-like optic neuropil receiving uncrossed axons from the medulla and equipped with large tangential neurons (shown at higher magnification in C) the axons of which project centrally. Scale bar = 100 μm.
Fig. 3.

Organization of retinula axons. A: Retinular cell axons (RCA) traversing the basement membrane (BM). Photoreceptor axons from one ommatidium meet those of neighboring ommatidia immediately above the basement membrane. Directly below the basement membrane, axons again fasciculate, the fascicles, separated by lacunae (L), each comprise axons from three ommatidia. B: Fascicles then coalesce into broader swathes of fiber bundles (FB) that cross the hemocoelic sinus to reach the lamina (see Fig. 1B). Before entering the lamina, just distal to the cell body layer (CBL), the swathes progressively split up into smaller bundles and finally into groups of eight axons. C: Each bundle of eight axons (upper right inset) enters the lamina where it defines an optic cartridge. There, a ring of 6 photoreceptor axons encircle a central one while the eighth is located outside the ring, as schematized in the lower right inset. Scale bar = 10 μm in A-C.
Fig. 4.
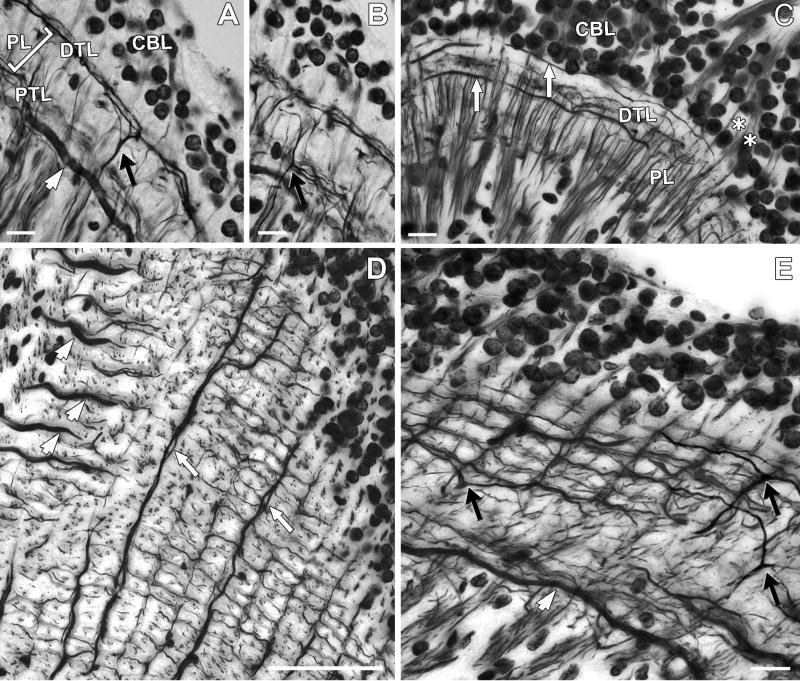
Architecture of the lamina. The lamina is composed of a plexiform layer (PL) and two layers formed by tangential processes, the distal tangential layer (DTL) and the proximal tangential layer (PTL). The neuropil is covered by a layer of monopolar neuron cell bodies (CBL). Reduced-silver method identifies the type 1 (black arrow) and type 2 (white arrowhead) lamina tangentials, as well as other elements, A,B: Transverse sections showing type 1 tangential processes extending in the distal (A) and in the proximal (B) tangential layer. Type 2 tangential processes have thick primary process situated in the PTL. Both tangentials extend along the lateromedial axis and cannot be seen in longitudinal sections, as in C, where type 3 tangential elements (white arrows) have long processes extending in the DTL just beneath the CBL. These processes extend along the anteroposterior axis and are not visible in transverse sections. The asterisks in C denote photoreceptor axon bundles entering the lamina. D: Tangential section showing the demarcation of retinotopic columns (optic cartridges). The processes of type 2 tangentials run perpendicularly to those of type 3, the main processes of which often occur as pairs (white arrows). The boundaries of the rectilinearly arranged optic cartridges seen in D (see also Fig. 3C), are defined by type 1 tangential processes as also shown in the oblique tangential section E. Black arrows mark the distal processes of three type 1 tangentials. Scale bar = 10 μm in A,B,C,E; 50 μm in D.
In malacostracans and insects, axons connecting the lamina with the medulla and the medulla with the lobula form chiasmata, which reverse and then re-reverse the anteroposterior order of the retinal mosaic (Fig. 2A). In certain insects, the lobula is accompanied by a tectum-like fourth neuropil, the lobula plate, which is characterized by wide-field tangential neurons and is linked to the medulla and to the lobula by uncrossed axons. Recently, the existence of a fourth optic neuropil linked to the medulla by uncrossed axons has been identified in the isopod Ligia occidentalis and the shrimp Palaeomonetes pugio (Sinakevitch et al., 2003). In Chasmagnathus, we identified a comparable neuropil anteromedially in the eyestalk located next to the lobula and just beneath the sinus gland (Figs. 1B,2B,C).
The Lamina
Eight axons from each ommatidium extend as a coherent bundle as far as the basement membrane of the retina. After penetrating the membrane (Fig. 3A), axons from triplets of ommatidia first converge and then further coalesce with other bundles to form large swathes of axons that cross a wide hemocoelic sinus (Fig. 1B) before again diverging as groups of eight axons that then enter their respective lamina cartridge (Figs. 3B,C). The overall appearance of retina-to-lamina projections is like that of the crab Leptograpsus variegatus, from which a meticulous electron microscopical study demonstrated that the retinal map is represented in the lamina by an unpermutated arrangement of retinula cells endings (Stowe, 1977).
The lamina is the best-studied visual neuropil (Hafner, 1973, 1974; Nässel, 1975, 1977; Nässel et al., 1978, Stowe, 1977; Stowe et al., 1977; Strausfeld and Nässel, 1980; Elofsson and Hagberg, 1986; Wang-Bennett et al., 1989; Glantz et al., 2000; Kleinlogel et al., 2003). As in insects, it is composed of four types of neurons. These are (1) receptor terminals from the retina; (2) monopolar cells and T-cell (efferent neurons) that carry information from the lamina to the medulla; (3) centrifugal cells from the medulla that terminate in the lamina; (4) local interneurons, or amacrine cells, that provide local circuits amongst elements of the lamina.
The lamina of Chasmagnathus (Fig. 1B) is an elongated structure with its long axis extended from the anterolateral to the posteromedial part of the eye. As in other described taxa, the lamina is composed of a synaptic layer surmounted by monopolar cell bodies (Figs. 2A,4A-C). The organization of its neuropil exemplifies that found throughout the optic lobes. Stratified arrangements of processes intersect through-going neurons. Monopolar cells provide axons that extend through a distal and proximal layer of tangential processes that derive from centrifugal cells extending out from the medulla (Fig. 4), and processes of amacrine cells that branch at both tangential strata in the lamina. Collateral branches of monopolar cells extend into these layers.
Although reduced-silver impregnation reveals neural architecture, it seldom allows convincing resolution of identifiable cell types. However, with reference to previous descriptions of Golgi-impregnated neurons, some cell types can be recognized in the present species. One is the type 1 tangential neuron (Tan 1), which in the crayfish has been described as providing from 3 to 6 primary branches from which extend numerous and bistratified secondary tangential processes that then further divide to form tertiary processes giving the whole arrangement a bush-like appearance (Nässel, 1977). The same organization of primary and secondary processes can be resolved in Chasmagnathus where distal and proximal processes provide two levels of stratification (Figs. 4A,B). But while Golgi impregnation shows up single tangential arborizations, reduced silver demonstrates that when all these cells are stained together as an ensemble they provide a regular plexus of rectilinearly arranged processes. Primary branches extend along the vertical axis whereas secondary branches extend anteroposteriorly, each branch extending alongside as many as 10 optic cartridges and overlapping with similar branches of other Tan 1 neurons (Fig. 4E).
Type 2 lamina tangentials have been previously described in the crayfish as possessing large primary processes situated just below the inner stratum at the level of outgoing monopolar axons to the first optic chiasma (Nässel, 1977). These primary tangential elements provide secondary branches that ascend distally into the lamina’s outer stratum. In Chasmagnathus the thick primary tangential processes of Tan 2 can be resolved in transverse sections (Fig. 4A), but not in longitudinal sections (Fig. 4C), thus showing that they are oriented exclusively along the lateromedial axis (Fig. 4D).
A third type of tangential neuron has long processes that extend almost exclusively along the anteroposterior axis in the outer stratum, just beneath the cell body layer (Fig. 4C). Likewise, because of their orientation such tangential processes do not show up in transversal sections. Frequently two processes, probably from two different cells, can be seen closely apposed extending alongside many cartridges (Fig. 4D). These fibers increase their diameter in the lateral side of the neuropil, where they then descend through the lamina’s plexiform layers to enter the first optic chiasma. We have here named these neurons type 3 tangential cells (Tan 3).
The Medulla
The medulla of Chasmagnathus is dome-shaped and slightly elongated in the lateromedial axis so that it appears wider frontally than laterally (Figs. 1B,2A,B). The medulla receives retinotopic information via the first optic chiasma (Fig. 2A) from the retina by R8 receptor axons and from the lamina by monopolar and T-cells. The few types of neurons that have so far been described for the medulla of any malacostracan species are almost exclusively from the crayfish (Strausfeld and Nässel, 1980) with the exception of identifiable GABA-immunoreactive elements that have been compared across in a variety of crustaceans (Sinakevitch et al., 2003).
Transmedullary neurons that terminate in the lobula derive from cell bodies above the medulla’s outer surface. A variety of T-neurons are provided by cell bodies beneath the medulla. Like transmedullary cells, these have dendrite-like processes in the medulla and terminals in the lobula. In addition to these cell types, all of which were described from Golgi impregnations, there are two types of wide field tangential neurons identified by Lucifer yellow injections (Kirk et al., 1982, 1983a) and tangential elements selectively revealed by antibodies against allostatin (Dircksen et al., 1999).
Reduced-silver preparations of the optic lobe of Chasmagnathus show that the medulla is organized into columns and layers (Fig. 2A). Crucially, the pattern of fiber staining observed in longitudinal sections appears quite different from that observed in transverse sections. This is because the organization of processes extending across columns is rectilinear: neurons extend processes either along the anteroposterior or the lateromedial axis. This can be seen by comparing figures 5A,B, where the layers defined by tangential processes extending along the lateromedial axis (Fig. 5A) appear as strata of profiles in sections cut parallel to the anteroposterior axis (Fig. 5B). Likewise, layers defined by tangential processes extending along the anteroposterior axis (visible in longitudinal sections, Fig. 5B), have corresponding strata formed by smaller profiles in transversal sections (Fig. 5A).
Fig. 5.
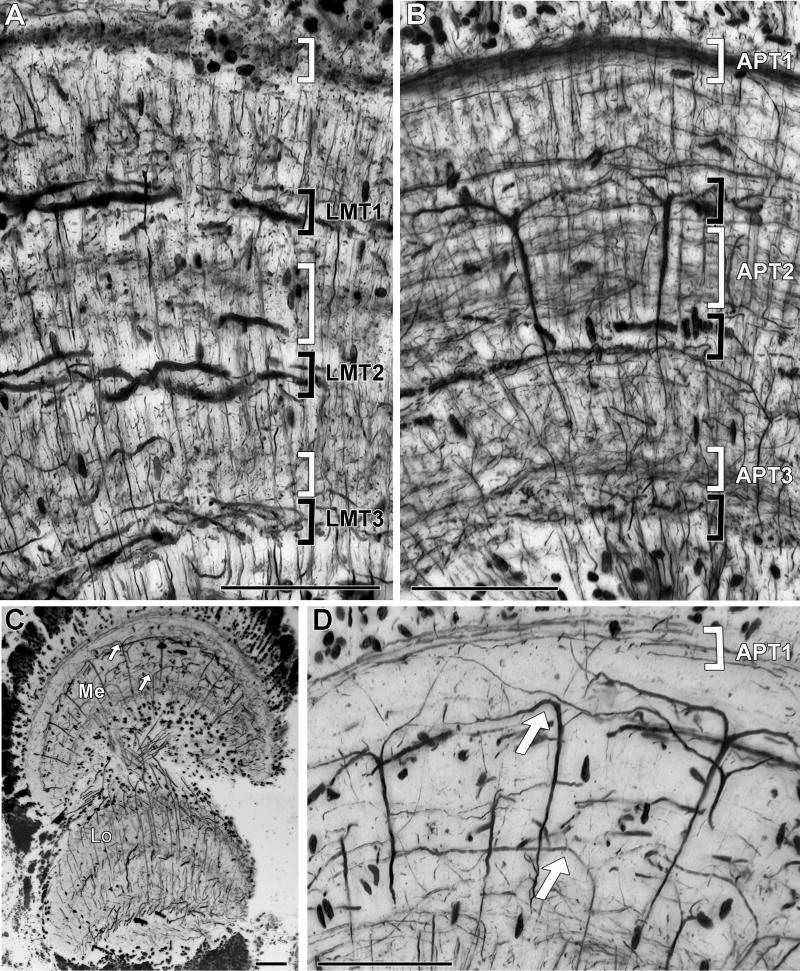
Neural architecture of the medulla. This neuropil comprises horizontal strata defined by tangential processes that within each stratum are oriented either in the anteroposterior or in the lateromedial axis. A: In transverse sections, tangentially directed processes are those oriented lateromedially (black brackets). Those oriented anteroposteriorly are seen as cross-sections (white brackets). From periphery to center the three lateromedial tangential strata are labeled LMT1, LMT2 and LMT3. B: Longitudinal section. In this plane, stratified tangential processes extend anteroposteriorly (white brackets) and lateromedial processes are observed as cross sections (black brackets). From periphery to center the three anteroposterior tangential strata are labeled APT1, APT2 and APT3. Additional tangential processes occur between the layers but do not form defined strata. C: Reduced-silver staining employing high copper concentrations suppresses staining of many cell types leaving only a few selectively resolved. Here longitudinal sections show arrays of Y-shaped branching transmedullary cells that link the medulla with deep levels in the lobula. Processes giving rise to the Y-shaped arborization are principally found at two levels (white arrows). D shows an enlargement of part of the medulla shown in C. Because the processes are oriented anteroposteriorly, these neurons are discernible only in longitudinal sections (see that they are present in B but not in A). Scale bar = 50 μm.
Transverse sections reveal three prominent strata defined by lateromedial tangential processes (Fig. 5A). These strata are separated by intermediate layers that are dominated by the thin dendritic processes of columnar elements and by slender tangential processes running anteroposteriorly. From the distal surface of the medulla to the inner (proximal) surface these layers have been annotated lateromedial tangential layers 1-3 (LMT1, LMT2, LMT3). LMT1 and LMT2 are formed by long wide-diameter processes whereas LMT3 is composed by comparatively shorter and thinner elements. Branches comprising LMT1 and LMT2 have a sinusoidal trajectory across retinotopic columns (Fig. 6). They increase in diameter towards the medial side of the medulla, where they originate from several large axons from the optic nerve (Fig. 6). Intracellular staining reveals that at least some of these arborizations correspond to sustaining and dimming neurons, which relay information directly to protocerebral centers in the brain (unpublished data).
Fig. 6.

Tangential section of the medulla showing the trajectories and tributaries of wide field tangential processes that thicken and converge towards the medial border of the neuropil (arrows). Scale bar = 100 μm.
Longitudinal sections reveal three thick strata that are defined by the anteroposterior trajectories of small-diameter tangential processes (Fig. 5B). From the more distal to the more proximal location these layers have been annotated as anteroposterior tangential layers 1-3 (APT1, APT2, APT3) respectively. APT1 comprises many densely packed fibers that run just beneath the cell body layer and which derive from perikarya above the medulla. This layer is thus interpreted as carrying mainly the neurites of transmedullary and amacrine cells. In contrast, APT2 and APT3 comprise a more diffuse arrangement of elements, the packing densities of which are revealed in transversal sections as differences in opacity (Fig. 5A).
A prominent feature of longitudinally cut sections is the ensemble of well-defined transmedullary cells that have prominent flattened Y-shaped bifurcations of their distal dendrites. Each of these neurons is thus obviously oriented along the anteroposterior axis of the retinotopic mosaic and their dendrites and axon originate from a slender neurite that arises from the outermost anteroposterior tangential layer (Fig. 5D). Although the axons of individual Y-shaped neurons could not be followed throughout the second optic chiasma in a single section, selective staining obtained by using high copper concentration in the reducing bath (see Methods) selectively reveals the retinotopic passage of their axons deep into the lobula (Fig. 5C).
The Lobula
Like the medulla, the lobula of Chasmagnathus is a dome shaped structure, slightly elongated in the lateromedial axis (Figs. 1B,7A,B). The lobula receives retinotopic information from the medulla via the second optic chiasma (Fig. 2A), one of its inputs being carried by neurons with Y-shaped medulla branches showed in figure 5C. These Y-shaped neurons terminate deep in the lobula. Their spacing in the medulla and lobula coarsens the retinotopic mosaic. Golgi studies (unpublished observations) show that this arrangement contrasts with the general architecture of both neuropils, in which most morphological types of through-going elements can be impregnated in neighboring retinotopic columns. Thus, unsurprisingly, reduced-silver preparations (Fig. 2A) show that the spacing of retinotopic columns across the lobula is approximately the same as that of columns across the medulla (compare Figs. 5A,B with Figs. 7A,B). Such an arrangement fits well with a model of the circuitry proposed to explain the binocular properties of lobula neurons in Chasmagnathus (Sztarker and Tomsic, 2004). Thus, in contrast to terrestrial insects (Strausfeld, 1998; Sinakevitch et al., 2003), the representation of the retinotopic mosaic is not coarsened in the crustacean lobula except by the spacing of certain relay neurons from the medulla.
Fig. 7.
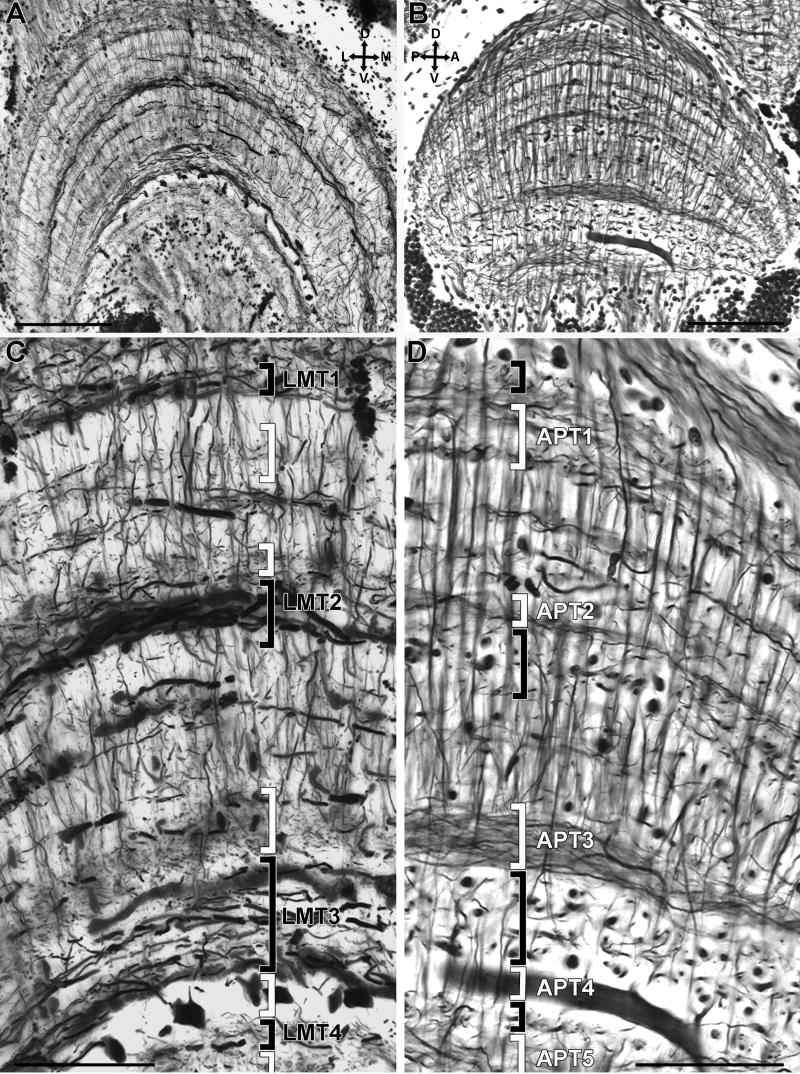
Neural architecture of the lobula. A transverse and B longitudinal sections. C and D are enlargements of A and B, respectively. Transverse sections reveal four strata (black brackets) comprising lateromedial tangential processes that are labeled LMT1-4 (see text). Lateromedial strata are separated by intermediate levels consisting of collaterals and terminal processes of columnar elements and by tangential processes extending orthogonally which appear as gray bands formed by various sized punctae (C, white brackets). These are seen in longitudinal sections where they define the anteroposterior strata APT1-5 (D, white brackets). The large diameter tangential trunk at level APT4 (B,D) is one of a set of 14 giant tangential neurons, some of which can be seen cut transversely in A and C where their large diameter profiles are regularly spaced between LMT3 and LMT4. Scale bar = 100 μm in A,B; 50 μm in C,D.
As in the lamina and the medulla, the fibroarchitectural appearance of the Chasmagnathus lobula depends on the section orientation because different cell types have their tangential processes exclusively oriented either along the anteroposterior or the lateromedial axis. Transverse sections show four strata of tangential processes oriented lateromedially. From the periphery to the center these strata are referred to as the 1st-4th lateromedial tangential layers LMT1-LMT4 (Figs. 7A,C). These strata are separated by regions containing arborization profiles belonging to columnar elements and local interneurons, and the profiles of tangential processes running anteroposteriorly. LMT1 and LMT4 comprise relatively thin tangential processes, whereas LMT2 and LMT3 contain long wide-diameter tangential fibers that increase in girth towards the medial side of the neuropil (Figs. 7A,8C). Additional tangential processes can be resolved between these strata, but they do not form defined levels. Intracellular staining (Fig. 8A,B) confirmed that LMT2 and LMT3 layers comprise the bistratified dendritic tree of wide field motion sensitive neurons termed bistratified movement detector neurons (B-MDNs) to distinguish them form the monostratified type described below. The dendritic tree of each of these neurons consists of several branches that run parallel to each other all along the lateromedial axis of the lobula. The dendrites converge towards the medial side of the neuropil into a thicker single axonal trunk that can be followed towards the midbrain (Berón de Astrada and Tomsic, 2002).
Fig. 8.
The lobula’s wide field motion detectors. A: Reconstruction based on several intracellular fills showing the anterior view of the structure of a bistratified movement detector neuron (B-MDN), as described by Berón de Astrada and Tomsic (2002). B: Transverse section of the lobula showing an intracellularly stained B-MDN with its typical bistratified branching pattern (brackets). C: In reduced-silver impregnated lobulas the bistratified branches (brackets) can be identified as forming part of layers LMT2 and LMT3. Towards the medial side of the neuropil these lateromedially oriented branches converge into a main trunk (see text). The proximal branches of B-MDNs are intercepted by 14 thick processes that extend normal to this plane of section. These are the primary processes of a system of 14 monostratified movement detector neurons (M-MDNs), which are oriented anteroposteriorly. D: At a higher magnification, secondary processes arising at right angles (white arrows) from the primary processes of M-MDNs, can be observed running parallel and close to the proximal arborizations of B-MDNs. Abbreviations: Lo, lobula; LPc, lateral protocerebrum; PcT, protocerebral tract. Scale bar = 100 μm in A,B,C; 10 μm in D.
Longitudinal sections of the lobula demonstrate five strata composed by tangential processes oriented anteroposteriorly. From the periphery to the center these are called the anteroposterior tangential levels APT1-APT5. The strata are separated by clearer layers in which the organization of columnar elements is apparent and from which arise processes that are oriented perpendicular to the plane of section.
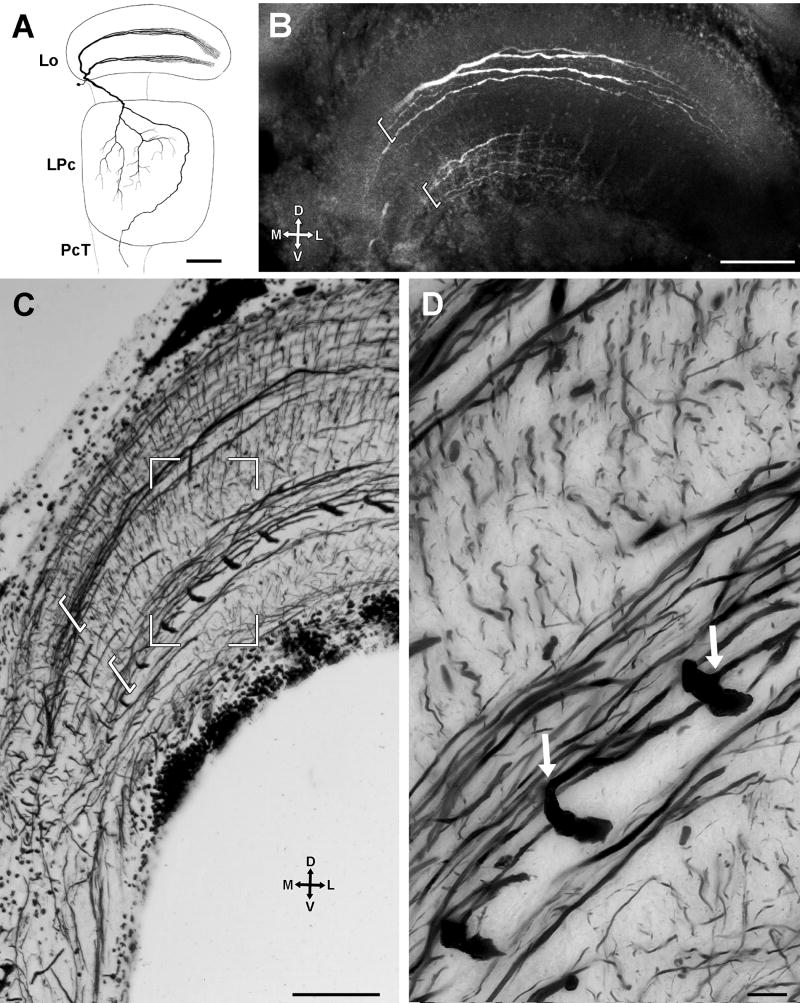
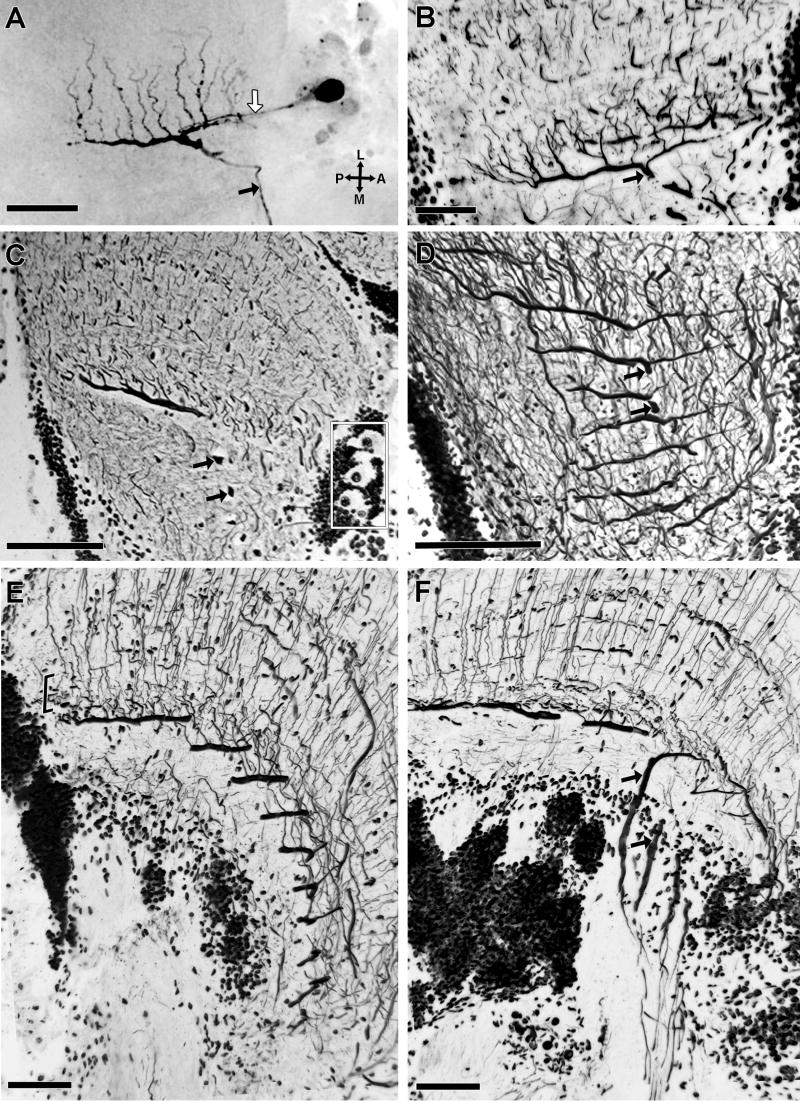
Level APT4 deserves especial attention being composed by approximately 14 neurons, each of which possesses an exceptionally wide-diameter primary branch (8-10 μm diameter) oriented along the anteroposterior axis (Figs. 7B,D, see also Fig 2A). Primary branches from fourteen cells are arranged in parallel, separated from one another by approximately 35 μm (Figs. 8C,9D). Each primary branch provides several secondary processes that arise at right angles and thus extend lateromedially (Figs. 9A,B) within the LMT3 stratum in close proximity to the proximal tangential processes of the B-MDNs (Figs. 8C,D,9E). Each primary branch is connected to a prominent axon that extends central for a short distance before being dramatically reduced in diameter after which it descends through the lateral protocerebrum to reach the optic tract (Fig. 9A). The descending axons of the approximately 14 neurons converge to form a discrete bundle (Fig. 9F). The large perikarya of these neurons are clustered anteromedially to the lobula (Figs. 9A,C) each being connected by a neurite to its primary branch (Fig. 9A). As will be described next, intracellular studies indicated that these cells integrate the group of motion sensitive MDNs described by Berón de Astrada and Tomsic (2002), therefore, hereafter they are termed monostratified movement detector neurons (M-MDN).
Fig. 9.
M-MDNs arborizations. A-D are tangential sections. E, F are slight oblique transverse sections. A: Intracellular staining of an M-MDN, revealing the thick main process running anteroposteriorly, from which secondary processes arise at right angle. The thin neurite connecting the soma to the main process (white arrow) and the descending axon from this process (black arrow) are also filled. B-F: reduced silver preparations. B: A section similar to that show in A, showing the same type of neuron but in a different species, the grapsid Hemigrapsus sanguineous. C: The 14 large cell bodies corresponding to the M-MDNs (4 of which are enclosed in the rectangle) are clustered anteromedially outside the neuropil. D: Parallel arrangement of primary processes from several M-MDNs. E: Terminal branches (bracketed) of transmedullary Y-branched neurons (see Fig. 5) appear clustered at the level of primary and secondary processes of M-MDNs, and the proximal branches of B-MDNs. F: Each axon of an M-MDNs that arises from a primary process in the lobula is initially thick (black arrow in this and previous panels) but then constricts before joining axons of the other M-MDNs to form a distinctive bundle. Scale bar = 100 μm in A,B,C,D; 50 μm in E,F.
Layer LMT3, which contains the secondary processes of M-MDNs and the proximal tangential processes of B-MDNs, is also supplied by the terminations of columnar Y-branched neurons that originate in the medulla (Figs. 9E,F, see also Fig. 5C).
DISCUSSION
The aim of this account has been to detail the neural architecture of the Chasmagnathus optic lobe. This is a highly visual grapsid crab, in which the neurobiology of learning and memory processes is being extensively investigated using behavioral, pharmacological and molecular techniques (for a review see Maldonado 2002). Intracellular recordings and dye filling can even be performed on the behaving animal, allowing the study of how visual processing by identified neurons relates to highly adaptive behaviors that involve long-term memory (Tomsic et al., 2003). Chasmagnathus therefore ranks as one of the most important arthropods for investigating the mechanisms of memory in relation to an adaptive behavior (e.g. Pedreira et al., 2002, 2004; Merlo et al., 2002, 2005; Pedreira and Maldonado, 2003; Tomsic et al., 2003). Surprisingly, however, very few studies on the anatomy of the visual nervous system of this or any other crab can be found in modern literature.
The optic neuropils of decapods
Traditional descriptions of malacostracan visual systems refer to three optic neuropils: the lamina, the external medulla (medulla), and the internal medulla (lobula). However, Strausfeld and Nässel (1980) describe in the crayfish the existence of additional smaller neuropils proximal to the lobula, which they called the optic foci by analogy with glomerular-like regions in insect protocerebra that receive lobula efferents. One of these optic foci (optic focus 4) was shown as receiving a retinotopic supply of uncrossed fibers from the medulla. This arrangement corresponds to recent descriptions of a fourth retinotopic neuropil in isopods and decapods that are claimed to be homologous to the insect lobula plate (Sinakevitch et al., 2003) and the entomostracan second optic neuropil, both of which are supplied by uncrossed retinotopic efferents (Strausfeld, 2005). In Chasmagnathus a fourth retinotopic neuropil is similarly supplied by uncrossed fibers from the medulla. It flanks the lobula in an anteromedial position just beneath the sinus gland. Reduced-silver staining shows the presence of neurons with large tangential processes and thick axons that leave this neuropil for the mid-brain (Fig. 2C).
The presence of a lobula plate neuropil in Crustacea has now entered the debate on the phylogenetic relationship amongst crustaceans and between malacostracans and insects (Sinakevitch et al., 2003; Strausfeld, 2005). The proposition that the optic neuropils of malacostracans and insects are homologous and are therefore a synapomorphy of the two groups would be additionally supported if they perform equivalent functions. In insects, the lobula plate is known to be involved in the control of optokinetic responses (Hausen, 1984; Borst and Haag, 2002) whereas in crustaceans the role of this neuropil is unknown. As expected for an optokinetic system, the large tangential neurons from the lobula plate of insects show sustained responses to continuous motion that characterizes optokinetic stimuli. While in the crab optokinetic behavior is easy to evoke and measure (Tomsic and Maldonado, 1990) there has been a singular failure to evoke responses to optokinetic stimuli from wide-field motion-sensitive neurons that lie deep in the lobula. Although many of these neurons react transiently to grating stimuli, they respond more vigorously to a single moving bar or edge (Medán et al., 2004). Moreover, upon repeated stimulation responses of these neurons rapidly decrement. Such results suggest that large tangential movement sensitive neurons recorded from the Chasmagnathus lobula are not involved in the control of optokinetic responses even though their large size and orthogonal arrangements of primary dendrites suggest comparison with giant vertical and horizontal cells of dipterous lobula plates. Thus, the possibility that optokinetic control in crustaceans is mediated by the fourth optic neuropil becomes appealing.
Neuroarchitecture of visual neuropils
Previous descriptions of decapod optic lobes (Nässel, 1975, 1977; Strausfeld and Nässel, 1980) confirmed classic studies by Hanström (1928) that crustacean optic neuropils are organized in columns and layers and that the organization of layers differs between longitudinal and transversal sections of the optic lobe. These differences arise because particular classes of cells have their tangential processes strictly oriented in a single direction. This is the case for many of the wide field tangential neurons found in the three optic neuropils, but also for smaller-field elements such as the type 1 lamina tangential cells and widely spaced columnar efferents, such as the class of Y-branched neurons linking the medulla with deep levels of the lobula. These arrays, such as those exemplified by the fibroarchitecture of the lamina, impart to each level of the lobes orthogonal arrangements of dendrites and terminals. To what extent these geometries reflect synaptic circuits is yet unknown and awaits both Golgi studies and electron microscopy.
Anatomical features of the lobula movement detector system
Movement detector neurons (MDNs) are so named because they preferentially respond to visual motion. MDNs share similar physiologies although it was recognized that these cells do not comprise a single morphological cell type. Intracellular staining showed that MDNs arborize extensively in the lobula and project toward the protocerebral ganglia. Possibly due to length of the optic stalk and thus the distance between the lateral protocerebrum and the midbrain, intracellular dye fills have so far failed to elucidate the morphology of these cells beyond the protocerebral tract. Arborizations of MDNs in the lobula revealed two morphologies; one monostratified, the other a bistratified system of tangential processes (Berón de Astrada and Tomsic, 2002). As shown here both can be readily recognized in reduced-silver preparations. Thus, despite the lack of physiological evidence that these two cells have different filter characteristics they are here obviously different cell types and have accordingly been annotated monostratified class M-MDNs and bistratified class B-MDNs.
Analyses of reduced-silver preparations reveal that M-MDNs comprise a population of 14 large neurons whose tangential processes are isomorphically distributed across the lobula mosaic. Each thick primary process extending along the anteroposterior axis gives rise to several secondary processes extending from it at right angles. These are arranged adjacent and parallel to the tangential processes of B-MDNs. Intracellular staining of an M-MDN reveals its large cell body surrounded by 13 other large somata, some of which show faint staining as well (Fig. 9A). Intracellular staining of a B-MDN can also result in faint staining of the primary processes of several M-MDNs suggestive of dye coupling (Fig. 8B). Recordings from single neurons having different spike amplitudes possibly reflect the activity of other MDNs electrically coupled to them (Berón de Astrada and Tomsic, 2002).
That B-MDNs and M-MDNs have not yet been distinguished by different physiological properties does not necessarily imply that differences do not exist. A variety of computer-generated visual stimuli presented to different parts of the crab’s visual field reveal two functional groups of MDNs: those that posses rather restricted receptive fields and pronounced directional preferences and those with receptive fields that subtend the entire panorama and lack directional sensitivity (Medán et al., 2004). Whether or not these two physiological groups correspond to the morphological divisions of M-MDNs and B-MDNs is still uncertain.
Comparative analysis
Crayfish, pelagic shrimps, and semiterrestrial crabs such as Chasmagnathus all occupy different ecologies and have very different lifestyles. As already noticed by Wiersma (Wiersma and Yamaguchi, 1967), semiterrestrial crabs react more readily to visual stimuli than do crayfish. The compound eye of Chasmagnathus has approximately 7000 ommatidia, which is about three times the number in adult crayfish. In crayfish, the eyes are horizontally oriented each side of the head so that the region of binocular vision is highly restricted to at most a small area of overlap. In contrast, the eyes of semiterrestrial crabs are mounted on vertically oriented stalks that when raised to their erect positions allow panoramic vision and thus binocular superposition (Sztarker and Tomsic, 2004). Do differences in the repertoire of visually evoked behaviors and differences in eye design of different species suggest differences in cellular organization of their optic lobes? Information available in the literature relates almost exclusively to the first optic neuropils where regardless of their different ecologies laminas are composed of conserved cell types (Strausfeld and Nässel, 1980; Elofsson and Hagberg, 1986). At that level, taxon-specific differences relating to the spread of terminal or dendritic arbors are likely to reflect neural circuits serving light adaptation.
Previous studies of Chasmagnathus (Berón de Astrada et al., 2001) have demonstrated neurons in the medulla that respond the same way as the sustaining and the dimming neurons identified in crayfish (Kirk et al., 1983a,b; Glantz and McIsaac, 1998; Glantz and Miller, 2002). Present observations of reduced-silver stained medullas, in combination with intracellular staining data (not shown), further suggest that sustaining neurons of Chasmagnathus are morphologically similar to those of crayfish (Kirk et al., 1983a). Taken together, these findings indicate that despite obvious differences in their habitats, lifestyles, and eye structure, some of the organization of the medulla may be similar across different decapod taxa. This may not be far-fetched if the lamina and the medulla serve fundamental visual processes such as the assessment of luminance and neural adaptation, visual noise elimination, early stages of motion computation (Glantz, 1994, 1996, 1998), and the segregation of chromatic and achromatic channels. Because these functions are likely to be ubiquitous across many malacostracan taxa these may share a subset of homologous neurons. There are, of course exceptions. In stomatopods, local differences of retinal organization and the ensemble of multichromatic channels at the eye’s equator are reflected in local modifications of lamina and medulla neuroanatomy (Kleinlogel et al., 2003). On the other hand, the lobula is likely to support higher perceptual as well as cognitive functions (Tomsic et al., 2003). In nature, visual cues that are crucial to survival differ for different species and it is likely that systems of matched filters deep in optic neuropils also show taxon-specific morphologies, as is the case for insects (Buschbeck and Strausfeld, 1997). In nature, the spatial and temporal parameters of behaviorally relevant visual stimuli relate to the ecological biology of each species. Individuals of Chasmagnathus live in large colonies, socially interact, and are subject to attack by aerial predators. They would require feature detector cells that serve behaviors triggered by configurations of motion stimuli very different from those that trigger evasive actions in a shrimp or crayfish. Comparisons amongst crustaceans suggest that lobula neuroarchitectures show interesting taxonomic variations (Strausfeld and Nässel, 1980; Sinakevitch et al., 2003; Strausfeld, 2005). Whether visually evoked behaviors typical of semiterrestrial crabs are due to the structural modification of existing cell lineages or the evolution of novel species of motion-sensitive neurons is a subject for further investigation that will require anatomical and physiological comparisons of Chasmagnathus both with sympatric and ecologically disparate species.
Acknowledgments
Grant sponsor: University of Buenos Aires, Grant number: EX172; National Research Council of Argentina (CONICET), Grant number: PIP736; Fundación Antorchas; Grant sponsor: National Institute of Health, Grant number: R01 RR08688.
Literature cited
- Backwell PR, Christy JH, Telford SR, Jennions MD, Passmore NI. Dishonest signalling in a fiddler crab. Proc R Soc Lond B Biol Sci. 2000;267:719–724. doi: 10.1098/rspb.2000.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berón de Astrada M, Sztarker J, Tomsic D. Visual interneurons of the crab Chasmagnathus studied by intracellular recordings in vivo. J Comp Physiol A. 2001;187:37–44. doi: 10.1007/s003590000174. [DOI] [PubMed] [Google Scholar]
- Berón de Astrada M, Tomsic D. Physiology and morphology of visual movement detector neurons in a crab (Decapoda: Brachyura) J Comp Physiol A. 2002;188:539–551. doi: 10.1007/s00359-002-0328-4. [DOI] [PubMed] [Google Scholar]
- Bodian D. A new method for staining nerve fibers and nerve endings in mounted paraffin sections. Anat Rec. 1937;69:153–162. [Google Scholar]
- Borst A, Haag J. Neural networks in the cockpit of the fly. J Comp Physiol A. 2002;188:419–437. doi: 10.1007/s00359-002-0316-8. [DOI] [PubMed] [Google Scholar]
- Buschbeck EK, Strausfeld NJ. The relevance of neural architecture to visual performance: phylogenetic conservation and variation in dipteran visual systems. J Comp Neurol. 1997;383:282–304. [PubMed] [Google Scholar]
- Cannicci S, Barelli C, Vannini M. Homing in the swimming crab Thalamita crenata: a mechanism based on underwater landmark memory. Anim Behav. 2000;60:203–210. doi: 10.1006/anbe.2000.1458. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Skiebe P, Abel B, Agricola H, Buchner K, Muren JE, Nässel DR. Structure, distribution, and biological activity of novel members of the allatostatin family in the crayfish Orconectes limosus. Peptides. 1999;20:695–712. doi: 10.1016/s0196-9781(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Duffy JE. The ecology and evolution of eusociality in sponge-dwelling shrimp. In: Kikuchi T, editor. genes, Behavior, and Evolution in Social Insects. Univ Hokkaido Press; Japan: 2002. pp. 1–38. [Google Scholar]
- Eckert MP, Zeil J. Towards an ecology of motion vision. In: Zanker JM, Zeil J, editors. Motion Vision: Computational, neural and ecological constraints. Springer Verlag; Berlin Heidelberg New York: 2001. pp. 333–369. [Google Scholar]
- Elofsson R, Hagberg M. Evolutionary aspects of the construction of the first optic neuropil (lamina) ganglionaris in Crustacea. Zoo-Morphology. 1986;106:174–178. [Google Scholar]
- Farris SM, Sinakevitch I. Development and evolution of the insect mushroom bodies: towards the understanding of conserved developmental mechanisms in a higher brain center. Arth Struct Devel. 2003;32:79–101. doi: 10.1016/S1467-8039(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Glantz RM. Directional selectivity in nonspiking interneurons of the crayfish optic lobe: evaluation of a linear model. J Neurophysiol. 1994;72:180–193. doi: 10.1152/jn.1994.72.1.180. [DOI] [PubMed] [Google Scholar]
- Glantz RM. Polarization sensitivity in the crayfish optic lobe: peripheral contributions to opponency and directionally selective motion detection. J Neurophysiol. 1996;76:3304–3414. doi: 10.1152/jn.1996.76.5.3404. [DOI] [PubMed] [Google Scholar]
- Glantz RM. Directionality and inhibition in crayfish tangential cells. J Neurophysiol. 1998;79:1157–1166. doi: 10.1152/jn.1998.79.3.1157. [DOI] [PubMed] [Google Scholar]
- Glantz RM, McIsaac A. Two-channel polarization analyzer in the sustaining fiber-dimming fiber ensemble of crayfish visual system. J Neurophysiol. 1998;80:2571–83. doi: 10.1152/jn.1998.80.5.2571. [DOI] [PubMed] [Google Scholar]
- Glantz RM, Miller CS. Signal processing in the crayfish optic lobe: contrast, motion and polarization vision. In: Wiese K, editor. The crustacean nervous system. Berlin: Springer-Verlag; 2002. pp. 486–498. [Google Scholar]
- Glantz RM, Miller CS, Nässel DR. Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. J Neurosci. 2000;20:1780–1790. doi: 10.1523/JNEUROSCI.20-05-01780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner GS. The neural organization of the lamina ganglionaris in the crayfish: a Golgi and EM study. J Comp Neurol. 1973;152:255–280. doi: 10.1002/cne.901520304. [DOI] [PubMed] [Google Scholar]
- Hafner GS. The ultrastructure of retinula cell endings in the compound eye of the crayfish. J Neurocytol. 1974;3:295–311. doi: 10.1007/BF01097915. [DOI] [PubMed] [Google Scholar]
- Hanström B. Vergleichende Anatomie des Nervensystems der wirbellosen Tiere. Berlin: Springer-Verlag; 1928. pp. 1–624. [Google Scholar]
- Harzsch S. The phylogenetic significance of crustacean optic neuropils and chiasmatha: a re-examination. J Comp Neurol. 2002;453:10–21. doi: 10.1002/cne.10375. [DOI] [PubMed] [Google Scholar]
- Hausen K. The lobula complex of the fly: Structure, function and significance in visual behavior. In: Ali MA, editor. Photoreception and vision in invertebrates. New York: Plenum Press; 1984. pp. 523–599. [Google Scholar]
- Hengstenberg R. Common visual response properties of giant vertical cells in the lobula plate of the blowfly Calliphora. J Comp Physiol. 1982;149:179–193. [Google Scholar]
- Hemmi JM, Zeil J. Robust judgement of inter-object distance by an arthropod. Nature. 2003;421:160–163. doi: 10.1038/nature01247. [DOI] [PubMed] [Google Scholar]
- Herberholz J, Issa FA, Edwards DH. Patterns of neural circuits activation and behavior during dominance hierarchy formation in freely behaving crayfish. J Neurosci. 2001;21:2759–2767. doi: 10.1523/JNEUROSCI.21-08-02759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk MD, Waldrop B, Glantz RM. The crayfish sustaining fibers. I. Morphological representation of visual receptive fields in the second optic neuropile. J Comp Physiol. 1982;146:419–425. [Google Scholar]
- Kirk MD, Waldrop B, Glantz RM. A quantitative correlation of contour sensitivity with dendritic density in an identified visual neuron. Brian Res. 1983a;274:231–237. doi: 10.1016/0006-8993(83)90700-x. [DOI] [PubMed] [Google Scholar]
- Kirk MD, Waldrop B, Glantz RM. The crayfish sustaining fibers. II. Response to illumination, membrane properties and adaptation. J Comp Physiol. 1983b;150:175–179. [Google Scholar]
- Kleinlogel S, Marshall NJ, Horwood JM, Land MF. Neuroarchitecture of the color and polarization vision system of the stomatopod Haptosquilla. J Comp Neurol. 2003;467:326–342. doi: 10.1002/cne.10922. [DOI] [PubMed] [Google Scholar]
- Land MF, Layne J. The visual control of behaviour in fiddler crabs: I. Resolution, thresholds and the role of the horizon. J Comp Physiol A. 1995;177:81–90. [Google Scholar]
- Lozada M, Romano A, Maldonado H. Long term habituation to a danger stimulus in the crab Chasmagnathus granulatus. Physiol Behav. 1990;47:35–41. doi: 10.1016/0031-9384(90)90039-7. [DOI] [PubMed] [Google Scholar]
- Maldonado H. Crustaceans as models to investigate memory illustrated by extensive behavioral and physiological studies in Chasmagnathus. In: Wiese K, editor. The crustacean nervous system. Berlin: Springer-Verlag; 2002. pp. 314–327. [Google Scholar]
- Medán V, Oliva D, Tomsic D. Preferences for direction of movement on visual neurons of a crab. Soc Neuroethol Abstr. 2004;7:142. [Google Scholar]
- Merlo E, Freudenthal R, Maldonado H, Romano A. The IkB kinase inhibitor sulfasalazine impairs long-term memory in the crab Chasmagnathus. Neurosci. 2002;112:161–172. doi: 10.1016/s0306-4522(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Merlo E, Freudenthal R, Maldonado H, Romano A. Activation of the transcription factor nf–kappab by retrieval is required for long-term memory reconsolidation. Learn Mem. 2005;12:23–29. doi: 10.1101/lm.82705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR. The organization of the lamina ganglionaris of the prawn, Pandalus borealis (Kroyer) Cell Tissue Res. 1975;163:445–464. doi: 10.1007/BF00218491. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Types and arrangements of neurons in the crayfish optic lamina. Cell Tissue Res. 1977;179:45–75. doi: 10.1007/BF00278462. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Elofsson R, Odselius R. Neuronal connectivity patterns in the compound eyes of Artemia salina and Daphnia magna (Crustacea: Branchiopoda) Cell Tissue Res. 1978;190:435–437. doi: 10.1007/BF00219557. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein Synthesis Subserves Reconsolidation or Extinction Depending on Reminder Duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Pérez-Cuesta L, Maldonado H. Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J Neurosc. 2002;22:8305–8311. doi: 10.1523/JNEUROSCI.22-18-08305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Pérez-Cuesta L, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extintion. Learn Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakevitch I, Douglass JK, Scholtz G, Loeser R, Strausfeld NJ. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J Comp Neurol. 2003;467:150–72. doi: 10.1002/cne.10925. [DOI] [PubMed] [Google Scholar]
- Stowe S. The retina-lamina projection in the crab Leptograpsus variegatus. Cell Tiss Res. 1977;185:515–525. doi: 10.1007/BF00220655. [DOI] [PubMed] [Google Scholar]
- Stowe S, Ribi WA, Sandeman DC. The organization of the lamina ganglionaris of the crabs Scylla serrata and Leptograpsus variegatus. Cell Tissue Res. 1977;178:517–532. doi: 10.1007/BF00219572. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Crustacean – insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav and Evolut. 1998;52:186–206. doi: 10.1159/000006563. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Evolution of Crustacean Optic Lobes and Origins of Chiasmata. Arthr Struct Dev. 2005 submitted. [Google Scholar]
- Strausfeld NJ, Nässel DR. Neuroarchitecture of brain regions that subserve the compound eyes of crustacea and insect. In: Autrum H, editor. Handbook of sensory Physiology. 6B. VII. Berlin: Springer Verlag; 1980. pp. 1–132. [Google Scholar]
- Sztarker J, Tomsic D. Binocular Visual Integration in the Crustacean Nervous System. J Comp Physiol A. 2004;190:951–962. doi: 10.1007/s00359-004-0551-2. [DOI] [PubMed] [Google Scholar]
- Tomsic . Visual learning in crabs investigated by intracellular recordings in vivo. In: Wiese K, editor. The crustacean nervous system. Berlin: Springer-Verlag; 2002. pp. 328–335. [Google Scholar]
- Tomsic D, Berón de Astrada M, Sztarker J. Identification of individual neurons reflecting short- and long-term visual memory in an arthropod. J Neurosci. 2003;23:8539–8546. doi: 10.1523/JNEUROSCI.23-24-08539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic D, Maldonado H. Central effect of morphine pretreatment on short- and long-term habituation to a danger stimulus in the crab Chasmagnathus. Pharmacol Biochem Behav. 1990;36:787–93. doi: 10.1016/0091-3057(90)90078-v. [DOI] [PubMed] [Google Scholar]
- Tomsic D, Massoni V, Maldonado H. Habituation to a danger stimulus in two semiterrestrial crabs. Ontogenic, ecological and opioid system correlates. J Comp Physiol. 1993;173:621–633. [Google Scholar]
- Tomsic D, Pedreira ME, Romano A, Hermitte G, Maldonado H. Context-US association as a determinant of long-term habituation in the crab Chasmagnathus. Ani Learn Behav. 1998;26:196–209. [Google Scholar]
- Wang-Bennett LT, Pfeiffer C, Arnold J, Glantz RM. Acetylcholine in the crayfish optic lobe: concentration profile and cellular localization. J Neurosci. 1989;9:1864–1871. doi: 10.1523/JNEUROSCI.09-06-01864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma CAG, Roach JLM, Glantz RM. Neural integration in the optic system. In: Sandeman DC, Atwood HL, editors. The biology of the crustacea, vol 4. Neural integration and Behavior. New York: Academic Press; 1982. pp. 1–31. [Google Scholar]
- Wiersma CAG, Yamaguchi T. Integration of visual stimuli by the crayfish central nervous system. J Exp Biol. 1967;47:409–431. doi: 10.1242/jeb.47.3.409. [DOI] [PubMed] [Google Scholar]
- Zeil J, Hoffmann M. Signals from ‘crabworld’: cuticular reflections in a fiddler crab colony. J Exp Biol. 2001;204:2561–2569. doi: 10.1242/jeb.204.14.2561. [DOI] [PubMed] [Google Scholar]