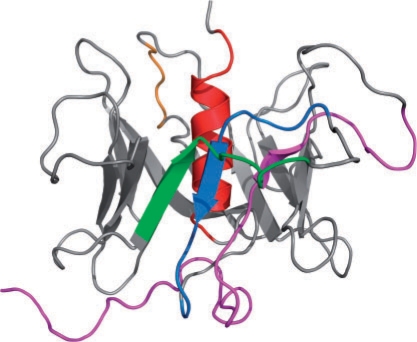
Fig. 2.
3D structural model of PLSCR1 computed by homology modeling. PLSCR1 forms a closed, symmetric β -barrel of 12 β-strands wrapped around a very hydrophobic C-terminal helix. Various sequence features of PLSCR1 are highlighted in color: transcriptional activation domain (magenta), Cysteine palmitoylation motif (orange), non-classical nuclear localization signal (green), Ca2+-binding motif (blue), predicted transmembrane helix (red).