Abstract
Ulcerative colitis (UC) is a dynamic, idiopathic, chronic inflammatory condition associated with a high colon cancer risk. American ginseng has antioxidant properties and targets many of the players in inflammation. The aim of this study was to test whether American ginseng extract prevents and treats colitis. Colitis in mice was induced by the presence of 1% dextran sulfate sodium (DSS) in the drinking water or by 1% oxazolone rectally. American ginseng extract was mixed in the chow at levels consistent with that currently consumed by humans as a supplement (75 p.p.m., equivalent to 58 mg daily). To test prevention of colitis, American ginseng extract was given prior to colitis induction. To test treatment of colitis, American ginseng extract was given after the onset of colitis. In vitro studies were performed to examine mechanisms. Results indicate that American ginseng extract not only prevents but it also treats colitis. Inducible nitric oxide synthase and cyclooxygenase-2 (markers of inflammation) and p53 (induced by inflammatory stress) are also downregulated by American ginseng. Mucosal and DNA damage associated with colitis is at least in part a result of an oxidative burst from overactive leukocytes. We therefore tested the hypothesis that American ginseng extract can inhibit leukocyte activation and subsequent epithelial cell DNA damage in vitro and in vivo. Results are consistent with this hypothesis. The use of American ginseng extract represents a novel therapeutic approach for the prevention and treatment of UC.
Introduction
Ulcerative colitis (UC) is a heterogenous, chronic and relapsing inflammatory condition that has a significant impact on the quality of life. Millions of people have this disease and have an increased colon cancer risk. Despite a wide variety of causes (e.g. environmental factors, genetic susceptibility and imbalanced enteric bacteria), the end result is an abnormal immune response with repeated episodes of colonic inflammation. While not everyone with colitis will develop colon cancer, risk increases when length of disease exceeds 10 years, on the order of 0.5–1.0% per year (1). Conventional treatment of colitis can reduce periods of active disease and help to maintain remission, but these treatments often bring marginal results, patients become refractory and there are side effects. For this reason, many colitis sufferers turn to unconventional treatments in hopes of abating symptoms of active disease and it is estimated that ∼40% of UC patients use some form of megavitamin therapy of herbal/dietary supplement (2,3).
The natural herbal American ginseng (Panax quinquefolius) improves mental performance and detrimental end points associated with diseases such as cardiovascular disease, diabetes and influenza (4,5). These diseases are all associated with inflammation, and American ginseng is a putative antioxidant in that it targets many of the key players involved in inflammation. Since UC is a chronic inflammatory disease, we hypothesized that American ginseng can be used to treat colitis. Here, we describe a role for American ginseng extract in the prevention and treatment of colitis.
Materials and methods
Chemicals and reagents
American ginseng extract was purchased from the National Research Council (NRC) of Canada, Institute for National Measurement Standards. The American ginseng roots were cultivated by Chai-Na-Ta Farms Ltd (Kamloops, British Columbia, Canada), which is the largest producer of North American ginseng in the world and alone accounts for 20% of global production. The ginseng obtained by NRC and processed by Canadian Phytopharmaceuticals Corporation (Richmond, British Columbia, Canada) was grown on the Harper Ranch, Kamloops, British Columbia, Canada, and obtained from the Harper 113 planting. The Harper 113 planting was made in 1999, emerged as seedlings in 2000 and was harvested as 4-year olds in the fall of 2003. Following grinding to pass 80 mesh, 35 kg of the root material was extracted with aqueous ethanol (75% ethanol and 25% water) in a recirculating filter extraction system for 4 h at a temperature of 60°C under vacuum. The ratio of solvent to root was 8:1 (vol/wt). After extraction, the filtrate was partially dried in vacuo to yield a concentrated extract; 2.8 kg of maltodextrin (40% of final weight) was then blended as a support and the resultant slurry was spray dried to yield 7 kg of free flowing powder. Analysis by Canadian Phytopharmaceuticals Corporation by High-performance liquid chromatography–ultraviolet against pure standards determined the total ginsenoside content (as the sum of Rg1, Re, Rb1, Rc, Rb2 and Rd) of the finished material to be 10.1% (wt/wt) and confirmed by High-performance liquid chromatography–mass spectrometry at the NRC of Canada. The final powder form of P.quinquefolius (American ginseng) extract supplied by Canadian Phytopharmaceutical Corporation contains (wt/wt) 10.1% ginsenosides (Rg1, Re, Rb1, Rc, Rb2 and Rd), 2% additional ginsenosides made up of F11, Ro, isomers of Rd and traces of malonyl ginsenosides and 40% of maltodextrin derived from hydrolyzed corn starch. The remaining 48% of the powder is made up of ginseng root-derived polysaccharides/oligosaccharides and proteins and up to 5% of moisture. The lot used was screened and found to be free of heavy metals and contaminants.
Proximate analysis of American ginseng conducted at the University of Guelph report a mean carbohydrate content of 73.4% and protein of 11.3%. The majority of the polysaccharide component is reported by the Ontario Ministry of Agriculture and Food to be starch. Using liquid chromatography-mass spectrometry, the NRC Canada conducted independent evaluation of the American ginseng extract used here, arriving at a marginally higher value of 11.1% total ginsenosides (wt/wt); mass fractions (mg/g) for each of the ginsenosides were found as follows: Rg1 3.0, Re 23.7, Rb1 44.2, Rc 15.9 and Rd 23.5. The NRC Canada values were determined using external standards that were obtained from Chromadex (Irvine, CA). Critically, liquid chromatography-mass spectrometry confirmed no detectable ginsenoside Rf characteristic of Asian ginseng and the presence of ginsenoside F11.
It should be noted here, that regular AIN-93M chow fed to mice contains 12.5000% maltodextrin. The addition of 75 p.p.m. American ginseng in the chow equates to 30 mg/kg final concentration of maltodextrin added to 12.5000% already in the chow. Therefore, there is 12.5000% maltodextrin in the AIN-93M chow and 12.5003% of maltodextrin in the AIN-93M chow supplemented with 75 p.p.m. American ginseng extract.
Cell culture and treatment
Cell lines were maintained in Dulbecco's modified Eagle's media (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Biofluids, Rockville, MD), 4 mM glutamine (Biofluids), penicillin (10 U/ml) and streptomycin (10 μg/ml, Biofluids). Experiments with American ginseng extract were carried out by preincubating cells with 200 μg/ml American ginseng extract for 12 h. A total of 200 μg/ml was chosen on the basis of its ability to inhibit inducible nitric oxide synthase (iNOS) levels by ∼50% (supplementary Figure 1A is available at Carcinogenesis Online) and that this level is non-toxic to the cell lines used here (supplementary Figure 1B and C is available at Carcinogenesis Online). Cells were washed before exposure to 100 U/ml interferon (IFN)-γ (R&D Systems, Minneapolis, MN; ANA-1 cells alone) or coculture of ANA-1 macrophages and colon cancer cells described as follows.
Coculture conditions
Coculture experiments were carried out as previously (6) with modifications. HT29 colon cancer cells were seeded at 2.5 × 106 cells per 150 mm culture dish 24 h before exposure to American ginseng (200 μg/ml) for 12 h. The American ginseng extract was washed off and then HT29 cells were exposed to activated or non-activated ANA-1 murine macrophages. Log-phase ANA-1 murine macrophages were activated with IFN-γ (100 U/ml; R&D Systems). ANA-1 cells were then added to the actively growing colon cancer cells at a 3:1 ratio (ANA-1:colon cancer cell). The coculture was incubated for time points indicated in figures before harvest. After harvest, HT29 and ANA-1 cells were separated with a MACS mini separator and CD45+ microbeads according to the manufacturer's instructions (Miltenyi BioTec, Auburn, CA). HT29 cells were then examined for DNA damage by Comet analysis; ANA-1 cells were examined for an oxidative burst by chemiluminescence.
Dextran sulfate sodium and oxazolone mouse models of colitis
An outline for all mouse models of colitis can be found in supplementary Figure 2 (available at Carcinogenesis Online). For our dextran sulfate sodium (DSS; MP Biomedicals, Solon, OH; 36 000–50 000 mw) mouse model, we followed the method of Seril et al. (7). Briefly, 8-week-old C57BL/6 mice received either water ad libitum or 1% DSS. All mice were on an AIN76A high iron diet. American ginseng extract was mixed into the chow of indicated groups at 75 p.p.m. (Research Diets, New Brunswick, NJ), which is the human equivalent dose of ∼58 mg daily. This is far below that used in previous clinical trials, where 1–3 g American ginseng has been shown to be effective in reducing blood sugar levels in patients with diabetes and healthy people (8,9). There are no significant side effects with long-term consumption of 3 g American ginseng (10). Our calculation of the human equivalent amount of American ginseng consumed by mice uses the body surface area normalization method (11) with the following assumptions: a typical mouse eats 3.5 g chow daily and weighs 22 g; the average adult human weighs 60 kg. More specifically, here, chow contains 75 p.p.m. American ginseng extract. This equates to 75 mg/kg of chow. A mouse consumes 3.5 g chow daily. Therefore, 75 mg/1000 g chow × 3.5 g chow/day = 0.2625 mg American ginseng extract daily. If a mouse weighs on average 22 g, then 0.2625 mg/22 g × 1000 g/1 kg = 11.93 mg/kg daily. As discussed by Reagan-Shaw et al. (11), the human equivalent dose (mg/kg) = animal dose (mg/kg) × (animal Km/human Km). As such, human equivalent dose (mg/kg) for mouse = 11.93 mg/kg/(3/37) = 0.967 mg/kg. If an average human adult weighs 60 kg, this equates to 0.967 mg/kg × 60 kg = 58 mg daily for humans. Mice consumed the same amount of chow daily (on average 3.5 g) regardless of it containing American ginseng extract (data not shown). We should note again here that the American ginseng extract we use contains 40% maltodextrin and equates to 12.5003% final amount in chow supplemented with 75 p.p.m. American ginseng extract. Chow without 75 p.p.m. American ginseng extract contains almost identical amounts of maltodextrin (12.5000% final conctration), making it unlikely any differences in groups are due to this ingredient.
To determine whether American ginseng extract prevents colitis onset, it was given to indicated groups of mice 1 week prior to and during DSS or water treatment. Organs were harvested from the treated mice after either 2.5 cycles (short-term treatment) or 14.5 cycles (long-term treatment), where each cycle in the DSS groups consisted of 1% DSS in drinking water for 7 days, followed by a 7 day interval (for short-term treatment) or a 10 day interval (for long-term treatment) with normal drinking water. To determine whether American ginseng extract can reverse/treat colitis, mice were fed DSS for 1.5 cycles (7 days DSS, 7 days water and 7 days DSS) and then given regular chow or chow containing American ginseng extract (75 p.p.m.). Mice were then euthanized at 1 cycle interval (another 7 days water and then 7 days DSS). For pathology and immunohistochemistry, colon tissue samples were washed with phosphate-buffered saline (PBS; Mediatech, Herndon, VA), cut longitudinally, swiss-roled, then formalin fixed and paraffin embedded. For western blot analysis, colonic epithelial cells were obtained from scrapings of full-length colon scrapings and immediately frozen at −80°C.
For our oxazolone model, we followed the methods described by Wirtz et al. (12). Briefly, mice were fed normal chow or chow containing American ginseng extract (75 p.p.m.) for 1 week prior to the initiation of the experiment. On day 0, the skin of the mice was treated with either 150 μl of oxazolone (Sigma, St Louis, MO) or 150 μl of vehicle control for presensitization. The oxazolone presensitization solution is four parts acetone to one part olive oil containing 3% (wt/vol) oxazolone. The vehicle control was four parts acetone to one part olive oil alone. After 1 week, mice were weighed, anesthetized and either 100 μl oxazolone solution or 100 μl vehicle control was given by rectal administration. The oxazolone solution was 1% oxazolone mixed into a 50% ethanol solution. The vehicle control was 50% ethanol solution alone. Mice were held in a vertical position (head down) for 60 s and then put back into their cages. After 4 days, mice were weighed, then euthanized and colons were processed as above for pathology by swiss-roll, fixation and paraffin embedding.
Immunohistochemical and immunofluorescence staining
For immunohistochemical staining, serial sections of mouse colon tissues (processed as described above) were incubated with antibodies against p53 (Mouse monoclonal, clone Pab 122, cat# X1494; diluted 1 in 1 million, Exalpha Biologicals, Maynard, MA), cyclooxygenase-2 (Cox-2) (Rabbit polyclonal, cat# 160126; diluted 1 in 20 000; Cayman Chemical, Ann Arbor, MI) or iNOS (Mouse monoclonal, clone 5D5-H7, cat# MC-5245; diluted 1 in 10 000; Research & Diagnostic Antibodies, North Las Vegas, NV). To ensure even staining and reproducible results, sections were incubated by slow rocking overnight in primary antibodies (4°C) using the Antibody Amplifier™ (ProHisto, LLC, Columbia, SC). Following incubation with primary antibody, sections were processed with EnVision+ System-HRP kits (DakoCytomation, Carpinteria, CA) according to the kit protocols. The chromogen was diaminobenzidene and sections were counter stained with 1% methyl green. The positive control tissue was colon cancer sections. Immunohistochemistry was quantified using the ACIS® III Automated Cellular Imaging System (DakoCytomation). ACIS® III consists of an automated bright-field microscope with image and proprietary processing analysis software for evaluating tissue sections on glass microscope slides. Results are represented as the percentage of cells staining positive.
For immunoflourescence, colons were harvested, fixed in freshly prepared 4% paraformaldehyde in PBS buffer (pH 7.2) and then vibratome sectioned at 100 μm. Tissue autofluorescence was quenched by incubation of the sections sequentially in PBS–glycine (150 mM) followed by NaBH4 (1 mg/ml in PBS). CD4+ cells were labeled with Alexa Fluor 488-conjugated rat anti-mouse CD4 monoclonal antibody (1:200, cat# 557667; Abcam, Cambridge, MA). Nuclei of all cells were labeled with 4′,6-diamidino-2-phenylindole (D3571, 1:5 000; Invitrogen, Carlsbad, CA). Finally, rhodamine phalloidin (Invitrogen; cat# R415, green) was used to stain for F-actin (Molecular Probes, Eugene, OR).
Generation of single-cell suspensions from mouse colons
In order to examine a leukocyte oxidative burst and associated epithelial cell DNA damage in vivo, separation of appropriate cell types was required. Accordingly, following treatment of mice for 2.5 cycles, we dissected out the colon, flushed it out with 1× PBS, opened it longitudinally and cut the colon into two pieces. Colons were incubated in 10% fetal bovine serum/5 mM ethylenediaminetetraacetic acid in 1× Ca2+/Mg2+-free PBS for 15 min at room temperature. Colon tissues were then shaken to dislodge the epithelial layer into single-cells suspensions. Cell viability was checked by trypan blue exclusion and >95% cells were viable. The single-cell suspension was centrifuged (1500 r.p.m., 5 min), and the pellet was brought up in freezing media and frozen at −80°C until Comet analysis.
The remaining lamina propria was added to an enzyme cocktail consisting of collagenase I (Sigma; 0.1 mg/ml) and dispase (EMD, Gibbstown, NJ; 0.2 mg/ml) for 75 min at 37°C in a shaking water bath. The suspension was further mechanically separated by pipetting and examined for trypan blue exclusion. Again, >95% of the single-cell suspension was viable. The suspension was passed through a MACS mini separator and CD45 microbeads according to the manufacturer's instructions (Miltenyi BioTec). Cells were counted and equalized to the same number (1 × 106 cells), centrifuged at 1500 r.p.m. for 5 min and then examined for an oxidative burst by chemiluminescence according to the manufacturer's instructions (World Precision Instruments, Sarasota, FL).
Quantifying inflammation
Slides were examined in a blind fashion by an experienced individual as described previously (13). Briefly, inflammation was graded by extent (focal, multifocal, diffuse or extensive areas) and depth/penetration of inflammation (lamina propria, into submucosa, into mucscularis propria and into subserosa). Ulceration/erosion was assessed by the overall extent in the colonic tissue. Both inflammation and ulceration/erosion were then given a numerical value of 0–4, where 0 is none observed, and 4 is severe inflammation and/or ulceration/erosion. A score of 5 was given when there was fulminant colitis and perforation leading to sepsis and death.
Western blot analysis and antibodies
Western blots were carried out as described previously (14). Antibodies used include: iNOS (Rabbit polyclonal, diluted 1 in 1000; Caymen Chemicals, Ann Arbor, MI), endothelial nitric oxide synthase (Rabbit polyclonal, diluted 1 in 500; BD Biosciences, San Jose, CA) and IkappaB-Phospho-Ser-32 (Rabbit polyclonal, diluted 1 in 1000; Cell Signaling Technology., Danvers, MA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Amersham Biosciences (Piscataway, NJ). Both secondary antibodies were diluted at 1:2000. All antibodies were diluted in 5% milk/PBST (1% Tween 20 in 1× PBS). Western blot signal was detected by Supersignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and developed onto Hyperfilm (Amersham Biosciences).
Nitrate and nitrite assay
Nitrite and nitrate are the stable end products of NO metabolism and are measured in culture media with a fluorometric assay kit as described by the vendor (Caymen Chemicals). Briefly, 10 μl of cell culture medium was incubated with nitrate reductase and enzyme cofactor for 1 h. For mouse colon tissue, 10 μl tissue homogenates were incubated for 2 h. Then, 2,3-diaminonaphthalene reagent was added to each well. After 10 min, NaOH was applied to neutralize the system. Results were read with FluoStar Galaxy (BMG, Durham, NC) fluorescence spectrometer (λex = 360 nm and λem = 430 nm).
Measuring an oxidative burst
An oxidative burst from inflammatory cells was measured by two methods. We first measured chemiluminescence according to the kit directions (World Precision Instruments). Briefly, collected inflammatory cells were washed and resuspended at 1 × 106 cells in Hank's buffered saline solution. Luminol and enhancer solutions were added and then placed in a luminometer, which quantifies an oxidative burst through the reaction of luminal with reactive oxygen species to produce a luminophore with an emission peak of 425 nm. Luminescence intensity is proportional to the amount of reactive species in the sample and is quantified as relative light units. We also examined reactive oxygen species through an Amage-iT™ detection kit (Molecular Probes), according to the kit directions. The technology is based on 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, a reliable fluorogenic marker for reactive oxygen species in live cells.
Comet analysis
An alkali Comet assay was performed according to instructions provided by the kit manufacturer (CometAssay™, Trevigen, Gaithersburg, MD). Cells treated with hydrogen peroxide (200 μM, 20 min.) were used as positive controls. A minimum of 50 Comets per treatment were quantified after capturing with and quantified by the Automated Comet Assay Analysis System (Loats Associates, Westminster, MD). Olive tail moment (15) was used to evaluate DNA damage. The tail moment, expressed in arbitrary units, is calculated by multiplying the percent of DNA (fluorescence) in the tail by the length of the tail in μm. The tail length is measured between the edge of Comet head and the end of the Comet tail. An advantage of using the tail moment as an index of DNA damage is that both the amount of DNA damage and the distance of migration of the genetic material in the tail are represented by a single number.
Statistical analysis
For prevention and treatment studies with inflammation and ulceration as an end point, a contingency table analysis was done on the DSS and DSS + American ginseng groups to determine if there is a statistically significant difference in their inflammation and ulceration scores. The use of a contingency table approach was motivated by the fact that the responses are categorical. Because of small sample sizes and a multitude of cells with zero frequencies, instead of computing the P-value using the chi-square distribution, we utilized a generalized Fisher's exact test approach. The use of the chi-square distribution in such cases will not provide a reliable estimate of the P-values. The exact P-values associated with the observed chi-square statistic values were computed using a method developed by Mehta et al., which are implemented in the statistical analysis system. For immunohistochemical quantification, mean differences between groups were compared by one-way analysis of variance with Scheffe multiple comparison tests. The P-value chosen for significance in this study was 0.05.
Results
American ginseng extract prevents DSS-induced colitis
There is an increasing evidence that American ginseng targets many key players in inflammation (5). UC is a high colon cancer risk, chronic inflammatory disease associated with overactive inflammatory cells infiltrating the colon. We therefore tested the hypothesis that American ginseng inhibits the onset of colitis. Table I shows results that are consistent with this hypothesis. One percentage DSS stimulates colitis. When American ginseng extract (75 p.p.m.) is given in the diet 1 week prior to and during short-term (2.5 cycles) and long-term (14.5 cycles) treatment with 1% DSS, mice are significantly protected from both inflammation (Table IA and B) and ulceration (Table IC and D) associated with colitis. The exact P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups (DSS versus DSS + American ginseng), is 0.0006 for the inflammation score, short-term treatment (Table IA); for the inflammation score the exact P-value was 0.0455, long-term treatment (Table IB); for the ulceration score the exact P-value was 0.0047, short-term treatment (Table IC) and for the ulceration score the exact P-value was 0.2030, long-term treatment (Table ID).
Table I.
Effects of American ginseng on the prevention of inflammation (A and B) and ulceration (C and D) in the colon induced by DSS
| Treatment group | Inflammation score |
Number of mice | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| A. Short-term treatment | |||||||
| Water | 7 (100)a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| Water + American ginseng | 7 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| DSS | 0 (0) | 0 (0) | 3 (42.9) | 3 (42.9) | 1 (14.2) | 0 (0) | 7 |
| DSS + American ginsengb | 2 (28.6) | 5 (71.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| B. Long-term treatment | |||||||
| Water | 15 (100)a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 15 |
| Water + American ginseng | 13 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 13 |
| DSS | 0 (0) | 0 (0) | 9 (30) | 6 (20) | 2 (6.7) | 13 (43.3) | 30 |
| DSS + American ginsengc | 1 (7.7) | 0 (0) | 9 (69.2) | 1 (7.7) | 0 (0) | 2 (15.4) | 13 |
| Treatment group | Ulceration score |
Number of mice | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| C. Short-term treatment | |||||||
| Water | 7 (100)a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| Water + American ginseng | 7 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| DSS | 0 (0) | 3 (42.9) | 1 (14.2) | 2 (28.7) | 1 (14.2) | 0 (0) | 7 |
| DSS + American ginsengd | 6 (85.8) | 1 (14.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 |
| D. Long-term treatment | |||||||
| Water | 15 (100)a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 15 |
| Water + American ginseng | 13 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 13 |
| DSS | 0 (0) | 7 (23.3) | 7 (23.3) | 2 (6.7) | 1 (3.4) | 13 (43.3) | 30 |
| DSS + American ginsenge | 1 (7.7) | 5 (38.5) | 2 (15.4) | 3 (23) | 0 (0) | 2 (15.4) | 13 |
Number in brackets indicates percentage of mice.
P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups (DSS versus DSS + American ginseng), is 0.0006.
P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups (DSS versus DSS + American ginseng), is 0.0455.
P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups (DSS versus DSS + American ginseng), is 0.0047.
P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups (DSS versus DSS + American ginseng), is 0.2030.
For the long-term experiment, we also observed that 14 mice died of severe intestinal perforations and associated sepsis. All 14 mice were from the 1% DSS-only group. Mouse weights and colon lengths were also measured upon euthanasia and indicated colon length in short-term treatment was decreased in the DSS group, but not in the DSS + American ginseng group.
Mouse colon length shrinks with stress, inflammation and ulceration. Therefore, as an additional indicator of inflammation and inflammatory stress, mouse colon lengths were measured upon euthanasia. For short-term (2.5 weeks) treatment, results indicate that compared with the colon lengths of the control (water treated) group (7.3 ± 0.2 cm), the length was significantly reduced in the DSS group (5.8 ± 0.6 cm). Mice consuming DSS + American ginseng had a non-statistically different colon length (7.1 ± 0.4 cm) to that of the water-treated group. For long-term (14.5 weeks) treatment, results also indicate that compared with the colon lengths of the control (water treated) group (7.5 ± 0.9 cm), the length was significantly reduced in the DSS group (4.8 ± 0.9 cm). However, in the long-term treatment, although the level of inflammation and ulceration was statistically and markedly improved by American ginseng, their colon length remained short (5.5 ± 0.5 cm). Interestingly, the ulceration/erosion contingency Table ID for the long-term data set showed insufficient evidence to conclude significant differences between the two groups for the ulceration response. Supplementary Figure 3 (available at Carcinogenesis Online) shows representative hematoxylin and eosin sections from the experiment.
Because CD4+ effector T cells play a role in driving colitis (13,16,17), we screened for infiltration of these cells as another end point of colitis. Supplementary Figure 3 (available at Carcinogenesis Online) also shows that mice consuming American ginseng have lower levels of CD4+ T cells observed in DSS-treated mice. Iron content is doubled in the chow of mice for this model because high iron diets are often encouraged for UC patients, and this stimulates a Fenton reaction so that less DSS is needed for generating colitis. We therefore examined the iron content of tissues and confirmed that all tissues had similar iron content (data not shown), eliminating the possibility that American ginseng extract sequesters iron as a mechanism toward tempering colitis.
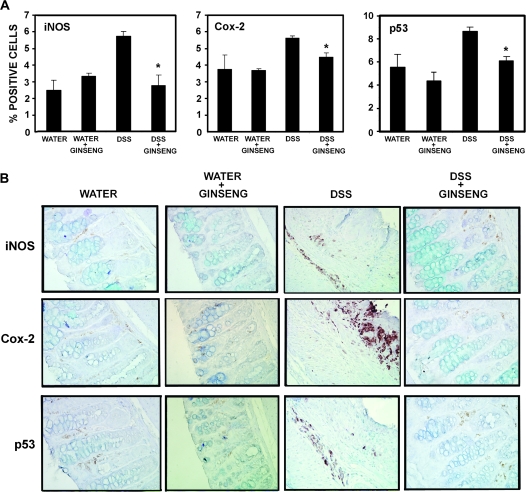
To further examine the impact of American ginseng on inflammatory markers, we examined iNOS and Cox-2. Because p53 is activated during inflammatory stress (6), we also probed tissue sections for p53. Immunohistochemical staining was accomplished by rocking slides using the Antibody Amplifier™ to ensure even, consistent and sensitive staining. Figure 1A shows quantification of staining, and results indicate that all three end points of inflammation (iNOS and Cox-2) and/or inflammatory stress (p53) are elevated in DSS-treated mice, but reduced to normal levels when DSS-treated mice are fed American ginseng (75 p.p.m.). Figure 1B shows representative serial sections of iNOS, Cox-2 and p53 from all four groups as indicated by labels.
Fig. 1.
American ginseng suppresses the players (18) involved in inflammation (iNOS and Cox-2) and inflammatory stress (p53). p53 (dilution: 1 in 1 million), Cox-2 (dilution: 1 in 20 000) and iNOS (dilution: 1 in 10 000) were examined by immunohistochemistry, using the Antibody Amplifier™, which allows the submersion and rocking of slides to ensure even staining and reproducible results. (A) Quantification of iNOS, Cox-2 and p53 as indicated (mean ± SEM). All three markers were elevated in the DSS-treated group and suppressed when the DSS-treated group were fed American ginseng (75 p.p.m.). *Indicates significant reduction in % positive cells in the American ginseng + DSS group compared with the DSS group. (B) Representative serial sections of markers of inflammation (iNOS and Cox-2) and inflammatory stress (p53) (×400 magnification).
American ginseng extract prevents oxazolone-induced colitis
DSS is directly toxic to gut epithelial cells of the basal crypts and affects the integrity of the mucosal barrier with the subsequent infiltration of immune (especially innate) cells (12). Intrarectal administration of the haptenating agent, oxazolone, leads to colitis with a Th2-polarized type of response, reminiscent of human UC (19,20). We therefore tested the hypothesis that American ginseng can inhibit colitis in this second model. Table II shows results that are consistent with this hypothesis. One percentage oxazolone stimulates colitis. When American ginseng extract (75 p.p.m.) is given in the diet 1 week prior to and during treatment with 1% oxazolone, mice are significantly protected from inflammation. The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0003 for the inflammation score. Ulceration did not occur in this model and therefore was scored as zero for all groups.
Table II.
Effects of American ginseng on the prevention of inflammation in the colon induced by oxazolone
| Treatment group | Inflammation score |
Number of mice | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Vehicle | 9 (100)a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 |
| Vehicle + American ginseng | 10 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 |
| Oxazolone | 0 (0) | 0 (0) | 5 (50) | 5 (50) | 0 (0) | 0 (0) | 10 |
| Oxazolone + American ginsengb | 0 (0) | 8 (80) | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 10 |
Number in brackets indicates percentage of mice.
The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0003 for the inflammation score. Ulceration did not occur in this model and therefore was scored as zero for all groups.
Mouse weights and colon lengths were also measured upon euthanasia and indicated that compared with the control (water treated) group (7.7 ± 0.7 cm), colon length was decreased in the oxazolone group (6.8 ± 0.3 cm), but not in the oxazolone + American ginseng group (8.1 ± 0.5 cm). Supplementary Figure 4 (available at Carcinogenesis Online) shows representative hematoxylin and eosin sections.
American ginseng extract reverses active colitis
To examine whether American ginseng extract can be used to treat colitis, we repeated the DSS experiment with timing modifications. Here, mice were given 1% DSS for 1.5 cycles (7 days DSS, 7 days water and 7 days DSS) and then fed either American ginseng extract (75 p.p.m.) or standard chow for the duration of the experiment. Table III shows results. One percentage DSS stimulates colitis. When American ginseng extract (75 p.p.m.) is given in the diet after 1.5 cycles, both inflammation and ulceration are reversed. The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0054 for the inflammation score. The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0088 for the ulceration score. Supplementary Figure 5 (available at Carcinogenesis Online) shows representative hematoxylin and eosin sections.
Table III.
Effects of American ginseng on treating inflammation and ulceration in the colon induced by DSS
| Treatment group | Inflammation score |
Number of mice | ||||
| 0 | 1 | 2 | 3 | 4 | ||
| DSS 1.5 cycles | 0 (0)a | 0 (0) | 2 (20) | 2 (20) | 6 (60) | 10 |
| DSS 2.5 cycles | 0 (0) | 0 (0) | 0 (0) | 5 (50) | 5 (50) | 10 |
| DSS + American ginseng 2.5 cycles | 0 (0) | 0 (0) | 2 (28.6) | 3 (42.8) | 2 (28.6) | 7 |
| DSS 3.5 cycles | 0 (0) | 0 (0) | 0 (0) | 5 (35.7) | 9 (64.3) | 14 |
| DSS + American ginseng 3.5 cyclesb | 0 (0) | 0 (0) | 5 (62.5) | 3 (37.5) | 0 (0) | 8 |
| Treatment group | Ulceration score | Number of mice | ||||
| 0 | 1 | 2 | 3 | 4 | ||
| DSS 1.5 cycles | 0 (0)a | 1 (10) | 6 (60) | 2 (20) | 1 (10) | 10 |
| DSS 2.5 cycles | 0 (0) | 0 (0) | 4 (40) | 5 (50) | 1 (10) | 10 |
| DSS + American ginseng 2.5 cycles | 0 (0) | 1 (14.3) | 6 (85.7) | 0 (0) | 0 (0) | 7 |
| DSS 3.5 cycles | 0 (0) | 2 (14.3) | 3 (21.4) | 5 (35.7) | 4 (28.6) | 14 |
| DSS + American ginseng 3.5 cyclesc | 0 (0) | 5 (62.5) | 3 (37.5) | 0 (0) | 0 (0) | 8 |
Number in brackets indicates percentage of mice.
The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0054 for the inflammation score.
The P-value associated with the observed chi-square value, under the hypothesis of no differences between the two groups, is 0.0088 for the ulceration score.
American ginseng extract inhibits the activation of inflammatory cells and associated DNA damage in target epithelial cells in vitro and in vivo
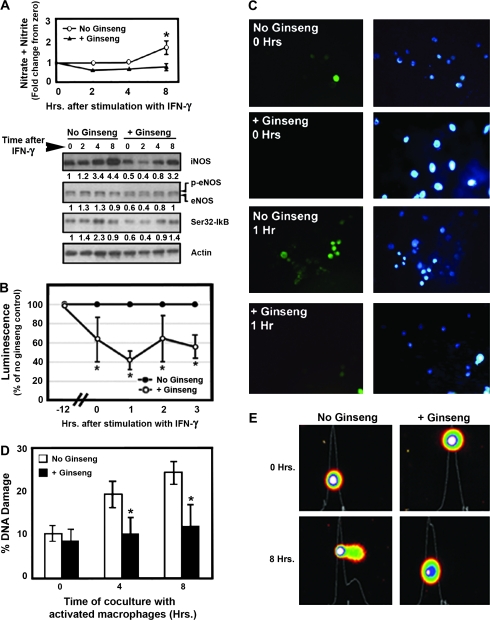
Mucosal and DNA damage associated with colitis is at least in part a result of an oxidative burst from overactive inflammatory cells (17,21,22). We therefore tested the hypothesis that American ginseng extract can inhibit leukocyte activation and resultant epithelial cell DNA damage. In vitro, we preincubated ANA-1 mouse macrophages with American ginseng extract (200 μg/ml, 12 h). This dose of American ginseng extract was chosen based on a dose response to inhibit iNOS (supplementary Figure 1 is available at Carcinogenesis Online). Following a 12 h incubation with American ginseng extract, cells were washed and then IFN-γ (100 U/ml media) was added to activate the macrophages. We first screened for protection from an inflammatory response by examining some key players in inflammation. We examined iNOS and endothelial nitric oxide synthase levels, nitric oxide release (nitrate/nitrite) and IκB phosphorylation (higher phosphorylation is suggestive of greater nuclear factor-kappa B (NF-κB) activity). Figure 2A shows that IFN-γ activates and American ginseng extract reduces the activation of all markers of inflammation, supporting the notion that American ginseng is an anti-inflammatory agent.
Fig. 2.
American ginseng extract attenuates the activation of macrophages and protects from DNA damage in target epithelial cells in vitro. (A) Nitrate/nitrite production, iNOS induction, endothelial nitric oxide synthase (eNOS) phosphorylation and IκB phosphorylation (representing NF-κB activation) following treatment of ANA-1 mouse macrophages with IFN-γ. *Overall, removing the effect of time, the American ginseng extract group had significantly lower nitrate/nitrite levels compared with the untreated (control) group (P < 0.05, analysis of covariance). Numbers below each blot represent the actin-adjusted density of each band. For endothelial nitric oxide synthase, the density of the upper (phosphorylated) band was examined because this represents an activated form of endothelial nitric oxide synthase. The observation that, for all three markers, density is lower in unstimulated cells exposed to American ginseng extract (0 h, +Ginseng, fifth lane) suggests that American ginseng extract inhibits basal activity of macrophages. Accordingly, it also inhibits the activation of macrophages. (B) An oxidative burst in ANA-1 mouse macrophages is attenuated by pretreatment with American ginseng extract (200 μg/ml). Chemiluminescence was measured as described in Materials and Methods. Results were compared with no ginseng control (±SEM). (C) Reactive oxygen species release, detected by fluorescence as described in Materials and Methods, is attenuated by pretreatment with American ginseng extract. Green fluorescence represents oxidatively stressed cells; blue fluorescence is cell-permeant nucleic acid stain Hoechst 33342. (D) In the presence of an oxidative burst, target epithelial cells (HT29 colon cancer cells) pretreated with American ginseng extract are protected from DNA damage. Results are represented as the mean Comet tail moment ± SEM, scoring a minimum of 50 Comets. (E) Representative photographs of Comets.
A consequence of the activation of macrophages is the release of free radicals in an oxidative burst. We therefore quantified an oxidative burst using chemiluminescence as described in Materials and Methods. Figure 2B shows that American ginseng extract inhibits an oxidative burst in cultured macrophages. Cells pretreated with American ginseng extract for 12 h have 62% of the oxidative burst capacity of cells not treated with American ginseng extract. This indicates that American ginseng extract blunts a basal oxidative burst. One hour after activation with IFN-γ, cells treated with American ginseng extract have 41% of the oxidative burst capacity of cells not treated with American ginseng extract, indicating that American ginseng extract also protects from an induced oxidative burst. Thereafter, cells begin regaining their oxidative burst capacity, presumably because of the depletion of American ginseng. Figure 2C highlights the ability of American ginseng extract to inhibit oxidative stress in ANA-1 macrophages. Green cells represent live cells releasing reactive oxygen species. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue).
Because an oxidative burst from macrophages can induce DNA damage in target epithelial cells, we cocultured the macrophages with colon cancer cells. We incubated HT29 colon cancer cells with or without American ginseng extract dissolved in media (200 μg/ml). We then washed the cells and added ANA-1 macrophages activated with IFN-γ as above at a 3:1 macrophage:HT29 cell ratio. Figure 2D shows a time-dependent increase in DNA damage, as assessed by a Comet assay. Cells preincubated with American ginseng extract were significantly protected from DNA damage at 4 and 8 h after the initiation of coincubation (P < 0.01). Figure 2E shows representative Comets.
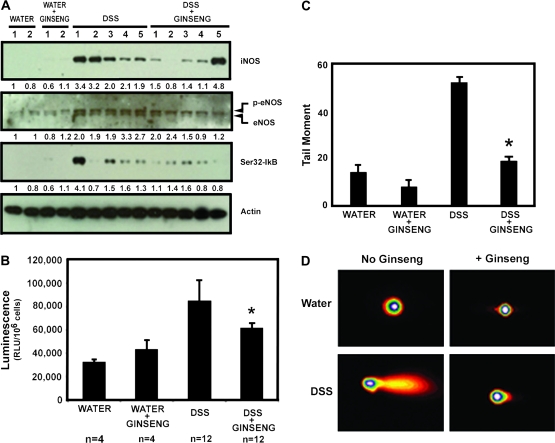
In order to test whether American ginseng extract inhibits an oxidative burst and associated DNA damage in vivo, we repeated experiments outlined for Table IA and C (short-term experiment) and then separated colon epithelial mucosa from the lamina propria as described in Materials and Methods. Figure 3A shows that DSS-treated mice have increased levels of iNOS, endothelial nitric oxide synthase and IκB phosphorylation (indicating NF-κB pathway activation). These end points are attenuated in the majority of mice consuming American ginseng extract. CD45+ cells were then examined for an oxidative burst by chemiluminescence as described in Materials and Methods. American ginseng extract suppresses the activity of CD45+ inflammatory cells in the colon, as measured by an oxidative burst (Figure 3B). Epithelial cells from the same mice were examined for DNA damage by Comet analysis. Figure 3C and D shows that DNA damage is blunted in mice consuming American ginseng extract. Taken together, these results indicate that American ginseng extract attenuates inflammatory cell activation and protects from target colon epithelial cell damage in vivo. Such results provide mechanistic reasoning for the ability of American ginseng extract to attenuate mucosal damage associated with colitis and the possibility of protecting from the increased risk of colon cancer.
Fig. 3.
American ginseng extract attenuates the activation of white blood cells and protects from DNA damage in target epithelial cells in vivo. Mice were either fed water ad libitum or 1% DSS in the drinking water for 2.5 cycles as described in Materials and Methods and in Table IA and C. (A) Protein lysates from scraped mucosa of the colon were examined for markers of inflammation. Numbers below each blot represent the actin-adjusted density of each band. For endothelial nitric oxide synthase (eNOS), the density of the upper (phosphorylated) band was examined because this represents an activated form of endothelial nitric oxide synthase. Mice consuming DSS only had activation of iNOS, endothelial nitric oxide synthase and IκB. Most mice consuming American ginseng extract and DSS had these markers attenuated. (B) Following column separation of inflammatory cells from mucosal cells, we examined an oxidative burst of CD45+ inflammatory cells. Mice consuming American ginseng extract exhibit CD45+ inflammatory cells with attenuated activity compared with mice on DSS only. Chemiluminescence was measured as described in Materials and Methods and expressed as mean (±SEM) relative light unit (RLU) per 1 × 106 cells. *Indicates significant difference from DSS-only group (P = 0.03). (C) Mucosal epithelial cells were examined for DNA damage by Comet analysis. Results are presented as the mean (±SEM) tail moment from 200 Comets taken from four mice per group. *Indicates significant difference from DSS-only group (P < 0.01). (D) Representative photographs of Comets.
Discussion
In recent years, there have been major advances and promising strategies for the treatment of inflammatory bowel disease (23). Conventional treatment of colitis can reduce periods of active disease and help to maintain remission. However, most treatments often bring side effects with marginal results and have population-specific efficacy. Many patients therefore turn to alternative treatment strategies, including Tai chi, probiotics, flax seed, aloe vera and garlic (2). The frequency of UC patients taking American ginseng is not currently documented in the literature. Given the ability of American ginseng to suppress key inflammatory players such as Cox-2, iNOS and NF-κB (5), we hypothesized that this agent will suppress colitis. Here, we show that American ginseng extract inhibits the onset of colitis (Tables I and II; Figure 1; supplementary Figures 3 and 4 are available at Carcinogenesis Online) and can be used to treat colitis (Table III; supplementary Figure 5 is available at Carcinogenesis Online). The doses we used of the extract were the human equivalent of 58 mg daily. This is significantly under that currently recommended for human consumption and doses used in clinical trials. In such trials, 1–3 g American ginseng has been shown to reduce blood sugar levels in both healthy and diabetic people (8,9,24). Four hundred milligrams American ginseng taken daily for 4 weeks reduced skeletal muscle membrane damage during exhaustive exercise (25). Although studies have shown that American ginseng does not appear to affect blood pressure, it is of note that such studies of long-term treatment also showed little side effects, indicating its safety up to 3 g daily (10,26). It appears that one of the few side effects are that American ginseng may reduce the effectiveness of the anticoagulant, warafin (27), and is therefore contraindicated when taking such drugs.
We also show that American ginseng extract suppresses the activation of key inflammatory markers such as iNOS and Cox-2 (Figure 1). These observations are consistent with other studies (6,28) and give insight into the anti-inflammatory mechanisms in vivo. Because p53 is a marker of inflammatory stress, we also probed for this end point. Our finding that p53 is also reduced indicates less inflammatory stress in animals receiving American ginseng extract. Because p53 appears to play a role in the protection from colitis by American ginseng, future experiments will focus on the role of p53 in inflammatory cell apoptosis. To this end, we have recently shown (13) that p53 overexpression in DSS-treated mice occurs mostly in inflammatory cells, providing a possible target for American ginseng as a mechanism to protection from overactive inflammatory cells.
Another interesting observation, represented in Figure 1, is that Cox-2 expression appeared in areas of high inflammation and ulceration near the luminal surface of the mucosa. Although iNOS and p53 expression also appeared in areas of high inflammation and ulceration, expression was mostly within the mucosa away from the luminal surface. The possibility that Cox-2 expression on the borders is due to drying effects can be ruled out because the slides were submerged in antibody solution and rocked during incubation (see Materials and Methods). The rationale for these expression patterns is currently under investigation. Interestingly, the observation that p53 and iNOS light up in similar areas is consistent with previous findings, showing that nitric oxide drives the overexpression of p53 in colitis (6). Overall, there is high expression of all three end points in the DSS-treated group, and in particular, in areas of mucosal damage.
UC and associated mucosal damage is at least partly caused by an increased number and hyperactive inflammatory cells in the colon (17). In this study, we asked whether American ginseng extract inhibits the activity of inflammatory cells and resultant epithelial cell DNA damage. In vitro experiments show that American ginseng extract can act on at least two levels in the colon. It can act at the level of inflammatory cells by inhibiting an oxidative burst. Alternatively, in the presence of an oxidative burst, it can directly protect epithelial cells from DNA damage. Noteworthy, we were also able to show that American ginseng extract reduces the activity of leukocytes and protects from damage to the colonic epithelial cell DNA in vivo. These results are consistent with in vitro studies from other labs. For example, red ginseng appears to protect cells from Helicobacter pylori-driven DNA damage and cytotoxicity (29). In animals, ginseng can inhibit micronucleus frequencies and chromosomal instability (30–32). It can also stimulate DNA repair (32). There is a strong link between chronic DNA damage and increased cancer risk. Based on results presented here, we are carrying out long-term separate studies to explore the hypothesis that American ginseng extract protects mice from inflammation-driven colon cancer.
In summary, we have shown American ginseng extract as a viable treatment strategy for colitis. Our data reveal that American ginseng directly inhibits leukocyte activation and DNA damage in target epithelial cells of the colon. Further studies will explore whether American ginseng extract can work upstream of the colon, in peripheral blood cells or lymphoid tissues. Indeed, other biological therapies can cause cellular apoptosis in the spleens of treated mice (33). A key mechanism for immune suppression is apoptosis of overly aggressive effector T cells and defects in mucosal T cell apoptosis is likely to play a key role in the pathogenesis of colitis (17,22). To this end, we recognize that there are several components of our American ginseng extract that can potentially influence the immune system. These not only include the ginsenosides (34) but also the polysaccharide/oligosaccharide components of the extract may have immunomodulatory activity (35). We can rule out an effect of maltodextrin since levels were almost identical between chows with and without the addition of American ginseng extract. Although, it may turn out that the most active extract is the combination of the active pharmacologic ingredients found in our American ginseng, ultimately the question of bioactivity of the extract with respect to colitis will be determined by bioassay-guided fractionation. Currently, however, we present evidence that the use of American ginseng extract represents a potential therapeutic approach for the abatement of signs and symptoms of inflammation associated with UC.
Supplementary material
Supplementary Figures 1–5 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R21DK071541-01 to L.J.H. and M.J.W.); Center of Excellence for Complementary and Alternative Medicine Research on Autoimmune and Inflammatory Disease (1P01AT003961-01A1 to P.N., L.J.H. and M.N.); Center of Biomedical Research Excellence funded University of South Carolina Center for Colon Cancer Research; National Institutes of Health (P20RR17698-01).
Supplementary Material
Acknowledgments
The authors thank the Statistical Core (Dr Edsel Pena, Director), Pathology Core (Dr William Hrushesky, Director), Administrative Core (Dr Frank Berger, Director), Mouse Core (Dr Marj Pena, Director) and Imaging/Histology Core supported by the Center for Colon Cancer Research. A special thanks is directed to the late Mrs Valerie Kennedy for her support in sectioning and scoring slides for immunohistochemical staining.
Conflict of Interest Statement: L.J.H. and A.B.H. are founders and owners of ProHisto, LLC. This company sells products used in immunohistochemistry procedures.
Glossary
Abbreviations
- COX-2
cyclooxygenase-2
- DSS
dextran sulfate sodium
- IFN
interferon
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear factor-kappa B
- NRC
National Research Council
- PBS
phosphate-buffered saline
- UC
ulcerative colitis
References
- 1.Itzkowitz SH, et al. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 2.Hilsden RJ, et al. Complementary and alternative medicine use by Canadian patients with inflammatory bowel disease: results from a national survey. Am. J. Gastroenterol. 2003;98:1563–1568. doi: 10.1111/j.1572-0241.2003.07519.x. [DOI] [PubMed] [Google Scholar]
- 3.Head K, et al. Inflammatory bowel disease. Part II: Crohn’s disease—pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2004;9:360–401. [PubMed] [Google Scholar]
- 4.Hofseth LJ. Ginseng and cancer. Healthy Aging. 2006:53–58. [Google Scholar]
- 5.Hofseth LJ, et al. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 6.Hofseth LJ, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc. Natl Acad. Sci. USA. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seril DN, et al. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig. Dis. Sci. 2002;47:1266–1278. doi: 10.1023/a:1015362228659. [DOI] [PubMed] [Google Scholar]
- 8.Vuksan V, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch. Intern. Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 9.Vuksan V, et al. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am. J. Clin. Nutr. 2001;73:753–758. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 10.Stavro PM, et al. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension. 2006;47:791–796. doi: 10.1161/01.HYP.0000205150.43169.2c. [DOI] [PubMed] [Google Scholar]
- 11.Reagan-Shaw S, et al. Dose translation from animal to human studies revisited. FASEB J. 2007;17:17. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 12.Wirtz S, et al. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 13.Kotakadi V, et al. Ginkgo biloba extract EGb 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis. 2008;28:1799–1806. doi: 10.1093/carcin/bgn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying L, et al. Chronic inflammation promotes retinoblastoma protein hyperphosphorylation and E2F1 activation. Cancer Res. 2005;65:9132–9136. doi: 10.1158/0008-5472.CAN-05-1358. [DOI] [PubMed] [Google Scholar]
- 15.Olive PL, et al. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the ‘comet’ assay. Radiat. Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 16.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007;149:173–186. doi: 10.1016/j.trsl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 18.Hofseth LJ, et al. Identifying and defusing weapons of mass inflammation in carcinogenesis. Biochim. Biophys. Acta. 2006;1765:74–84. doi: 10.1016/j.bbcan.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Kawada M, et al. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J. Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strober W, et al. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 21.Luhrs H, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002;37:458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 22.Baumgart DC, et al. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 23.Targan SR. Current limitations of IBD treatment: where do we go from here? Ann. N. Y. Acad. Sci. 2006;1072:1–8. doi: 10.1196/annals.1326.032. [DOI] [PubMed] [Google Scholar]
- 24.Vuksan V, et al. American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J. Am. Coll. Nutr. 2000;19:738–744. doi: 10.1080/07315724.2000.10718073. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CC, et al. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J. Gastroenterol. 2005;11:5327–5331. doi: 10.3748/wjg.v11.i34.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stavro PM, et al. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension. 2005;46:406–411. doi: 10.1161/01.HYP.0000173424.77483.1e. [DOI] [PubMed] [Google Scholar]
- 27.Yuan CS, et al. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: a randomized, controlled trial. Ann. Intern. Med. 2004;141:23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- 28.Nam SY, et al. DA-6034, a derivative of flavonoid, prevents and ameliorates dextran sulfate sodium-induced colitis and inhibits colon carcinogenesis. Exp. Biol. Med. (Maywood) 2008;233:180–191. doi: 10.3181/0707-RM-186. [DOI] [PubMed] [Google Scholar]
- 29.Park S, et al. Rescue of Helicobacter pylori-induced cytotoxicity by red ginseng. Dig. Dis. Sci. 2005;50:1218–1227. doi: 10.1007/s10620-005-2763-x. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova T, et al. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem. Toxicol. 2006;44:517–521. doi: 10.1016/j.fct.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Panwar M, et al. Inhibition of benzo(a)pyrene induced lung adenoma by Panax ginseng extract, EFLA400, in Swiss albino mice. Biol. Pharm. Bull. 2005;28:2063–2067. doi: 10.1248/bpb.28.2063. [DOI] [PubMed] [Google Scholar]
- 32.Rhee YH, et al. Inhibition of mutagenesis and transformation by root extracts of Panax ginseng in vitro. Planta Med. 1991;57:125–128. doi: 10.1055/s-2006-960047. [DOI] [PubMed] [Google Scholar]
- 33.Fuss IJ, et al. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology. 1999;117:1078–1088. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 34.Rivera E, et al. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine. 2005;23:5411–5419. doi: 10.1016/j.vaccine.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Predy GN, et al. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173:1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.