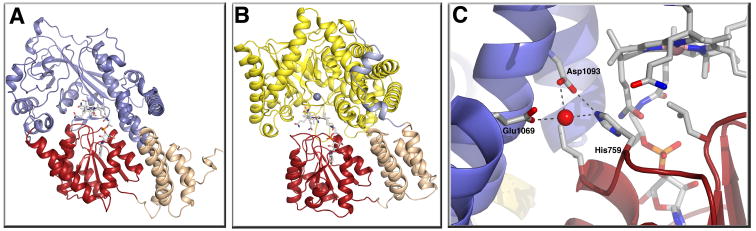
Figure 3. (A) Structure of the reactivation (AdoMet:Cob) conformation of methionine synthase[10••].
This structure was obtained with a fragment of the enzyme containing only the cobalamin-binding and adenosylmethionine-binding modules. The AdoMet-binding module is shown in blue, the cobalamin-binding domain in red, and the cap in tan. To reduce the conformational flexibility yet at the same time keeping the active site intact, a strategy of disulfide cross-linking between the two modules was used. Two cysteine mutations, Ile690Cys (in the cap domain) and Gly743Cys (in the cobalamin-binding domain) were introduced. The resulting disulfide cross-link sufficiently favored the conversion from His-on to His-off such that the protein crystallized in the AdoMet:Cob conformation. Nε of His-759 has moved 5.6 Å away from the cobalt towards the AdoMet binding module and is involved in an intermodular hydrogen bonding contact. (B) Structure of the MtaBC complex [27••] of methanol:coenzyme M methyltranferase, shown in the same orientation as methionine synthase in (A). MtaB forms a decorated TIM barrel (yellow), with the zinc atom (gray sphere) located at the C-terminal opening of the barrel in close proximity to the corrinoid of MtaC. The zinc is ligated by two cysteines and a glutamate, while the identity of the fourth ligand remains unclear. The sequences containing these three ligands bear no resemblance to sequences associated with zinc binding in other family members, despite the fact that the closest structural relative of MtaB is the homocysteine-binding domain of methionine synthase. Methanol is not present in the MtaBC complex, but if coordinated to the zinc by its hydroxyl oxygen, could be positioned appropriately for methyl transfer to the cobalt of the 5-hydroxybenzimidazolyl cobamide of MtaC, MtaC consists of two domains, a corrinoid-binding domain (red) and a four helix bundle or cap (tan) with an N-terminal extension (light blue). (C) Details of the intermodular interaction of His-off methionine synthase. His759 forms a hydrogen bond to Glu1069 and a water mediated hydrogen bond with Asp1093. This contact is expected to stabilize the His-off form in the AdoMet:Cob conformation.