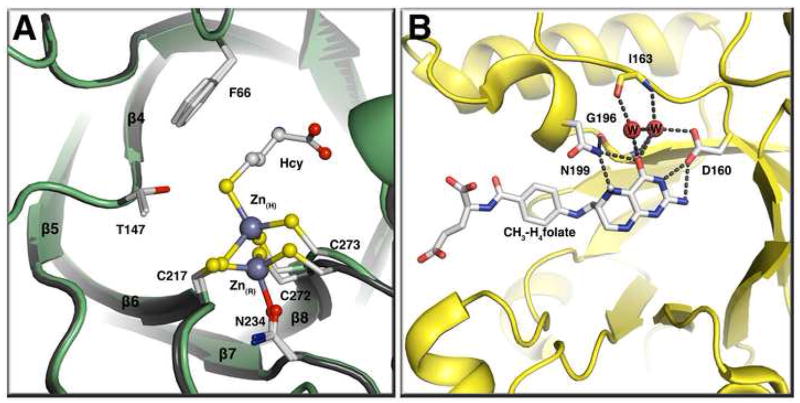
Figure 4. (A) The homocysteine binding domain of methionine synthase in the presence or absence of homocysteine[14**].
This panel shows a tructure superposition of the MetH resting state (gray) and the Hcy bound state (green) with Zn(R) representing the zinc atom at the resting state (no substrates bound) and Zn(H) the zinc atom at the Hcy bound state. Binding of homocysteine leads to an inversion of geometry at the active-site zinc and the displacement of Asn234 from the zinc coordination sphere. The zinc moves 2 Å on binding of homocysteine. (B) The CH3-H4folate-binding domain of the corrinoid iron-sulfur protein methyltransferase AcsE [41•]. CH3-H4folate is unprotonated in this structure and N5 accepts a hydrogen bond from Asn199.