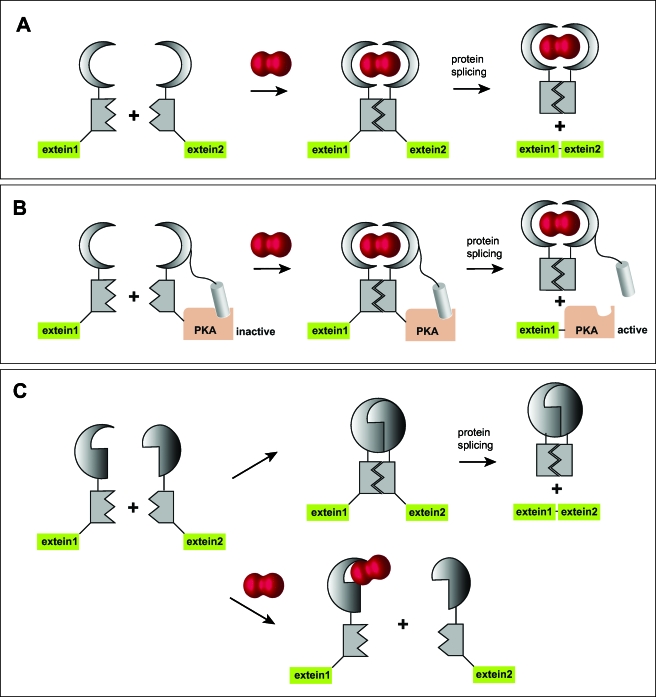
Figure 8. Rapamcyin-induced protein splicing.
By using a split intein with each half fused to one of the rapamycin-binding proteins FKBP and FRB, respectively, a functional intein is generated as soon as rapamycin is present, leading to the generation of the protein of interest (a). Alternatively, an inhibitor peptide may be fused to half of the intein carrying the protein of interest, here protein kinase A. The kinase is activated as soon as rapamycin is permitting protein splicing, resulting in the separation of the inhibitor peptide and the now active kinase (b). Finally, the procedure can be reversed by using dimerizing mutants of rapamycin-binding proteins that constantly produce the protein of interest. Rapamycin addition will in this case inhibit protein synthesis (c).