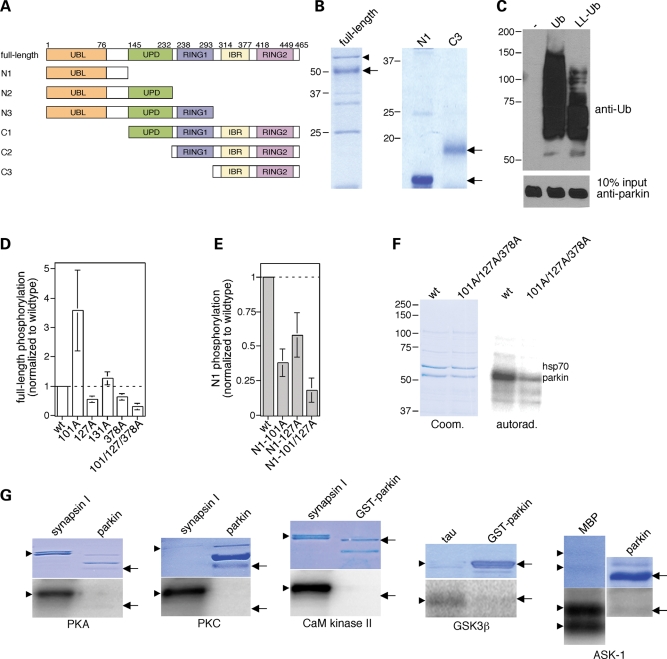
Figure 1.
Parkin phosphorylation by casein kinase I in vitro. (A) Schematic representation of parkin domain structure, with domain boundaries shown by amino acid residue numbers above, and of recombinant parkin domain combinations (full-length, N1–N3 and C1–C3) analyzed by in vitro phosphorylation assays. (B) The different recombinant parkin domains were purified as described in Materials and Methods, and analyzed for purity by SDS–PAGE and Coomassie staining. Full-length recombinant parkin as well as N1 and C3 truncated forms are indicated by arrows. Bacterial hsp70 (arrowhead), as determined by mass spectroscopy, co-purified with full-length parkin. (C) Full-length recombinant parkin was catalytically active, as assessed by in vitro autoubiquitylation in the presence of wild-type ubiquitin (Ub) or a lysine-less derivative (LL-Ub) lacking the conjugation sites necessary for polyubiquitylation. When the reaction was performed with LL-Ub, lower levels of ubiquitylation were detected, indicating that recombinant parkin displays both multiple monoubiquitylation as well as polyubiquitylation activity towards itself. (D) Full-length recombinant parkin (wt), or equal amounts of the indicated point-mutated versions (S101A, n = 4; S127A, n = 4; S131A, n = 4; S378A, n = 4; S101A/S127A/S378A, n = 3), were subjected to in vitro phosphorylation reactions using casein kinase I, and phosphate incorporation quantified by using a PhosphorImager (mean±S.E.M.). Note that the S101A mutant displayed increased phosphate incorporation in the context of the full-length protein, possibly reflecting conformational effects. (E) The N1 fragment of recombinant parkin (wt) or equal amounts of the indicated point-mutated versions (S101A, n = 6; S127A, n = 4; S101A/S127A, n = 2) were subjected to in vitro phosphorylation reactions using casein kinase I, and phosphate incorporation quantified by using a PhosphorImager (mean±S.E.M.). (F) Example of a phosphorylation experiment using full-length recombinant parkin (wt) or the triple mutant S101A/S127A/S378A. (G) Parkin phosphorylation by additional protein kinases in vitro. Full-length recombinant parkin or glutathione S-transferase-parkin (arrows) was subjected to in vitro phosphorylation reactions using cAMP-dependent protein kinase (PKA), PKC, Ca2+/calmodulin-dependent protein kinase II (CaM kinase II), GSK3β or ASK-1, as indicated, using various proteins as positive controls (synapsin, tau or MBP, respectively) (arrowheads). None of these kinases significantly phosphorylated parkin in vitro.