Fig. 2. Three-dimensional comparison of ORC indicates ATP gives rise to analogous conformational changes in the core of ORC independent of phosphorylation state. All volumes were filtered to 30 Å and are displayed in four identical views rotated 90 ° counter-clockwise around their elongated axis.
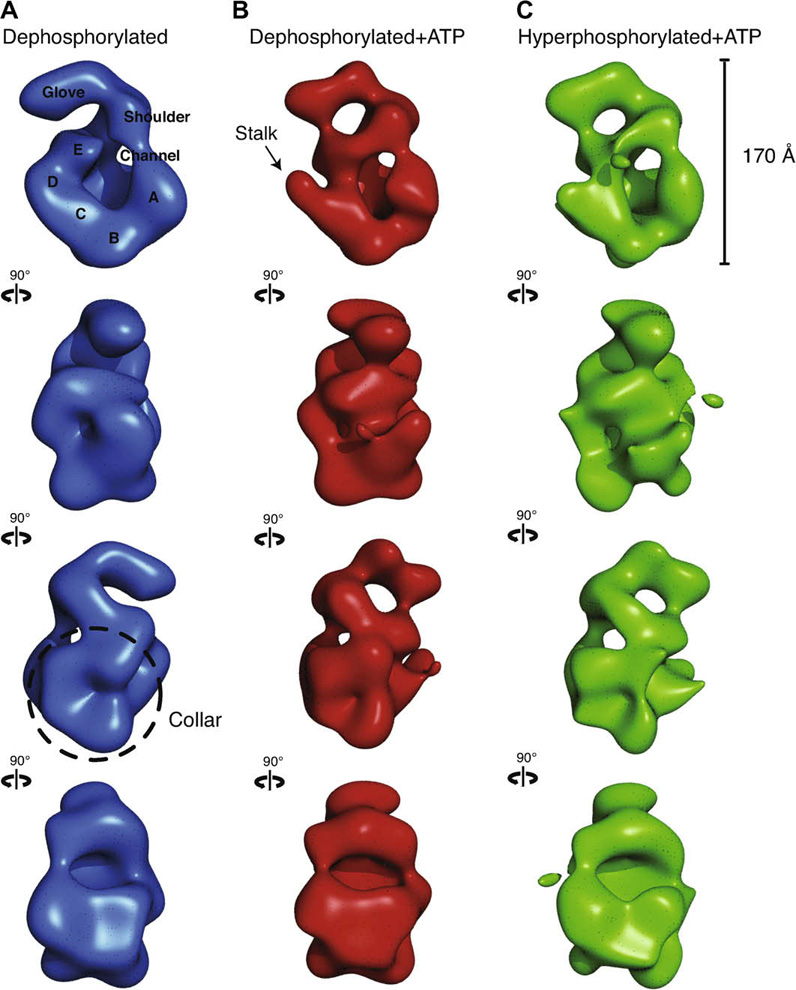
(A) Dephosphorylated ORC is rendered as a blue isosurface. The five lobes of density composing the core are labeled A–E. A thin density closes the central channel and fuses the shoulder to lobe A. The glove density at the top remains separate from the main body of the complex. (B) Dephosphorylated ORC bound to ATPγS is rendered as a red isosurface. An arrow points to the region where upon nucleotide-binding a discernable stalk density separates lobe D from E. The shoulder is again fused with lobe A and the glove is now connected to lobe E. The density labeled lobe E was previously identified as Orc5 by immuno-labeling. (C) Hyper-phosphorylated ORC bound to ATPγS is rendered as a green isosurface. The separation of lobes D and E as well as the fusing of the glove with lobe E is also observed, indicating that nucleotide binding promotes a very similar conformational state in the core of ORC regardless of the phosphorylation state of the complex.
