Fig. 5. Speculative model of ORC subunit architecture and the path of 133 bp of DNA.
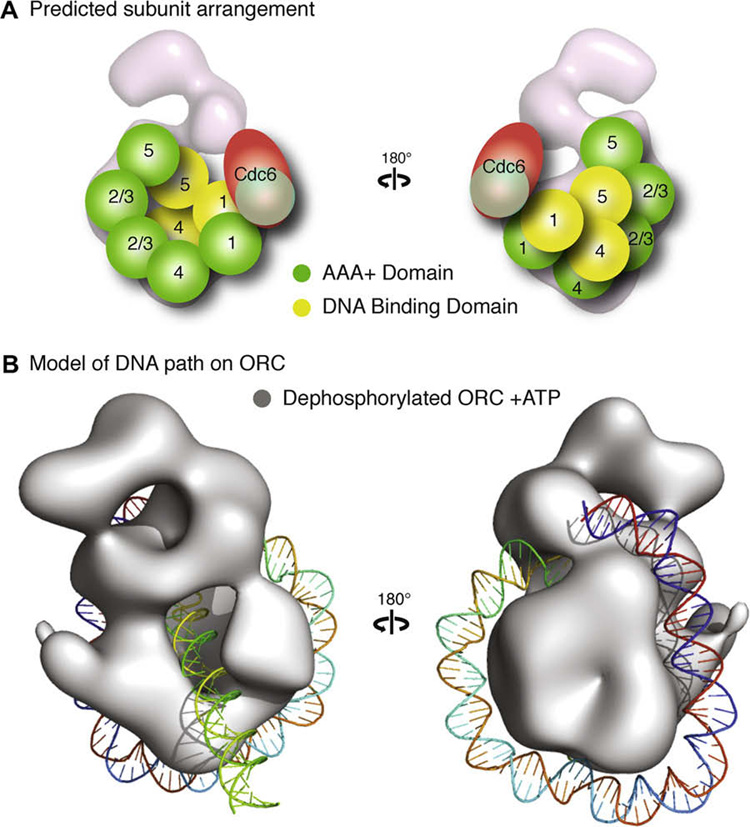
(A) A potential arrangement of the subunits would have the five AAA+ domains (illustrated as green spheres) of Orc1–5 composing the core of the complex, with Orc1 situated at the base directly contacting Orc4, Orc2 and Orc3 in the central region where the stalk density shifts upon nucleotide binding, followed by Orc5. The position of Orc2 and Orc3 are interchangeable and are labeled “2/3” to indicated this in the figure. The winged helix domains (illustrated as yellow spheres) of Orc1, 4 and 5 would form the collar. This position would allow for the AAA+ domain of Cdc6 to dock at the AAA+ interface of Orc1. The mass of the other domains of Orc1–5 and Orc6 would compose the remainder of the density. (B) A model of dephosphorylated ORC bound to ATPγS (rendered as a grey isosurface) with 133 bp of a linear DNA wrapped around the complex starting at the wing-helix domains in the collar and following a right-handed path along the AAA+ backbone to loop back through the channel.
