Abstract
This paper describes a simple approach to the synthesis of asymmetric, hybrid colloidal particles by precipitation polymerization. The key is to introduce Au or Ag colloids 2 min after (rather than before) starting the polymerization. The hybrid particles were uniform in size, and each one of them only contained one Au (or Ag) nanoparticle in its surface. Due to the simplicity of this procedure, it should be possible to use it for large-scale production. This method can be extended to metal nanoparticles other than Au and Ag and with a range of sizes, as long as they have appropriate charges on the surface.
Increasing attention has been paid to colloidal particles with an asymmetric, hybrid structure because of their use in applications such as fabrication of optical, optoelectronic, and sensing devices through the “bottom up” approach.1 Many efforts have been devoted to the synthesis of asymmetric, hybrid colloidal particles, and approaches include surface selective modification,2 template-assisted self-assembly,3 phase separation,4 surface-controlled nucleation and growth,5 and microfluidic techniques.6 In spite of the success, it is worth pointing out that most of these methods cannot be easily applied to large-scale production because of the two-dimensional strategy or multiple steps involved in a typical fabrication process. Still, a facile and robust procedure is desired for the preparation of asymmetric, hybrid colloidal particles.
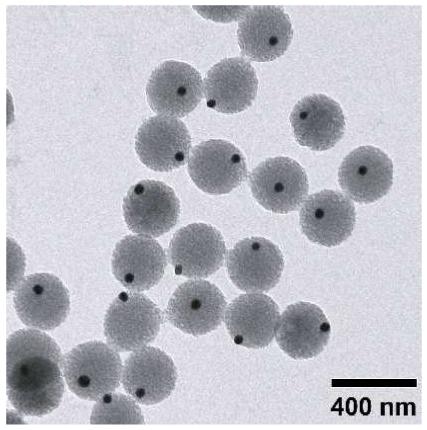
Here we report a simple and versatile procedure for generating asymmetric, hybrid particles consisting of metal nanoparticles and polymer beads. We simply modified precipitation polymerization of polystyrene (PS) by adding Au (or Ag) colloids into the system after the polymerization had proceeded for a few minutes. The asymmetric particles were uniform in size and morphology, with each PS bead containing only one Au or Ag nanoparticle in its surface. Scheme 1 shows a schematic illustration of the procedure we used for generating the Au-PS asymmetric, hybrid particles. We firstly added styrene and divinylbenzene (DVB) to a mixture of ethanol and water, in which 4-styrenesulfonic acid sodium salt (NaSS) and potassium persulphate (KPS) had been dissolved. Since NaSS and KPS could stabilize the growing PS beads, we did not introduce other surfactants into the reaction system. After 2 min into the synthesis at 70 °C, we introduced Au colloids (50 nm in diameter) into the reaction mixture. At this point, PS oligomers and/or monomers started to nucleate by adsorbing onto the surface of Au nanoparticles, then grew in size, and eventually evolved into spheres as confined by surface tension. After 4 h into the reaction, the final product of hybrid particles was harvested by centrifugation and repeated washing with a mixture of ethanol and water. Figure 1 shows SEM and TEM images of the as-prepared Au-PS asymmetric, hybrid particles. The particles were slightly nonspherical in shape, but uniform in size with an average diameter of 300 nm. Essentially every PS bead contained one Au nanoparticle partially embedded in the surface. In order to make sure that each PS bead had only one Au nanoparticle in its surface, we calculated the number of particles from the dried weight, the densities, and the sizes of Au nanoparticles and PS beads for the sample shown in Figure 1. We found that the number of PS beads (∼1.5×1011) was essentially the same as the number of Au nanoparticles (∼1.4×1011) added into the reaction system.
Scheme 1.
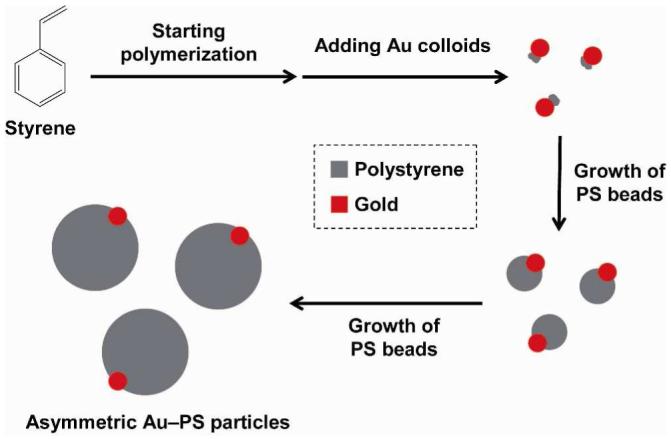
A schematic illustration of the procedure for generating asymmetric Au-PS colloidal particles.
Figure 1.
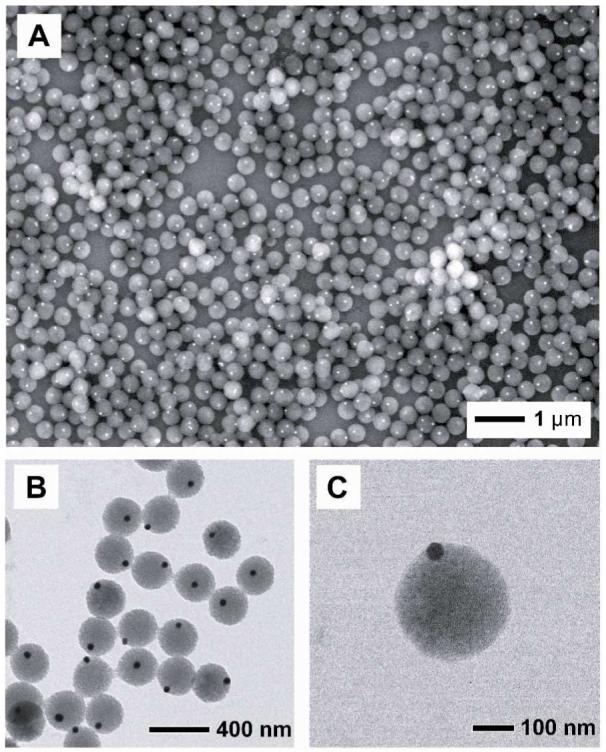
(A) SEM and (B, C) TEM images of the Au-PS asymmetric particles. The average diameter of the Au nanoparticles and the PS beads were 50 and 300 nm, respectively.
From Figures 1B and 1C, it is clear that the Au nanoparticle slightly protruded from the surface of the PS bead, not located in the center of each particle as seen in the work of Kamata et al.7 This difference implies that the PS bead grew anisotropically from the surface of each Au nanoparticle during the polymerization process. To decipher the growth mechanism, we investigated the evolution of Au-PS asymmetric particles as a function of reaction time. Figure 2A shows TEM image of an intermediate product sampled 5 min into the reaction or 3 min after the Au colloids was added. It can be seen that a PS bump was formed on the surface of the Au nanoparticle through a heterogeneous nucleation process. As polymerization continued, the PS nucleus gradually grew into larger dimensions as shown in Figure 2B (40 nm, 10 min into the reaction), Figure 2C (150 nm, 30 min), Figure 2D (200 nm, 45 min), and finally Figure 1 (300 nm, 4 h). Interestingly, the Au nanoparticle was not encapsulated in the center by a uniform polymer shell as the PS bead grew in size. Instead, the Au nanoparticle maintained at the surface of the PS bead and being slightly embedded in the surface (Fig. S1).
Figure 2.
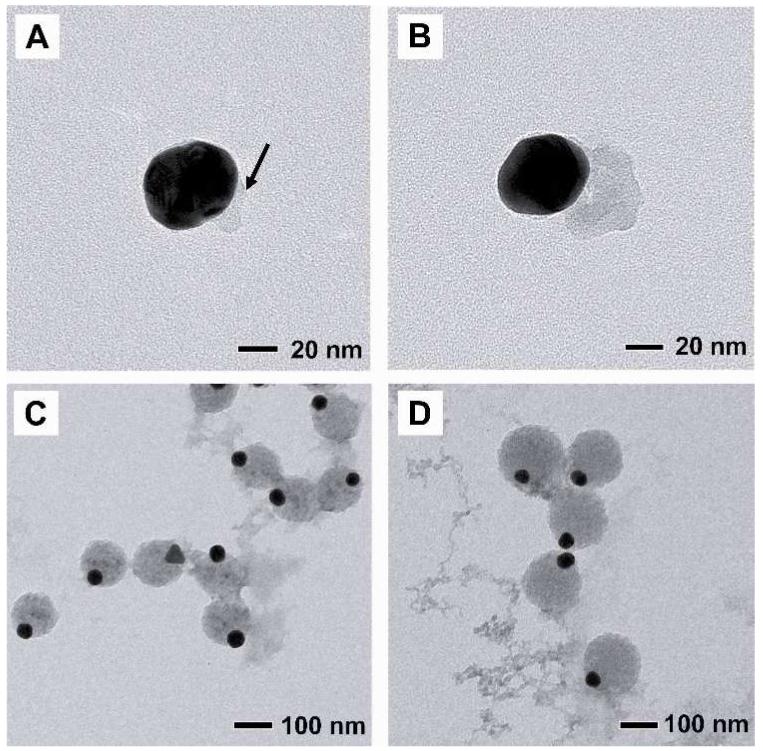
TEM images of the reaction intermediate obtained at (A) 5 min (the black arrow indicate PS), (B) 10 min, (C) 30 min, and (D) 45 min, respectively, after the initiation of polymerization. The PS bead was growing from one side of the Au nanoparticle as the polymerization was continued.
From a set of experiments, we found that the Au colloids had to be added at a right time (e.g., 2 min into the reaction as in Fig. 1) in order to obtain the asymmetric, hybrid particles. If Au colloids were added before polymerization, the PS bead might contain a large number of Au nanoparticles due to aggregation, which was likely induced by the initiator and other ionic species (Fig. S2). If Au colloids were added 30 min after starting polymerization, the yield of hybrid particles dropped to ∼50% (Fig. S3). These results suggest that the PS oligomers formed in the early stage of polymerization could stabilize the Au colloids and thus prevent agglomeration. In addition, it seems to be that the PS oligomers at t=2 min were only able to nucleate and grow on the surface of Au nanoparticles, rather than in the solution through a homogeneous pathway. This explains why the sample shown in Figure 1 had a yield close to 100%. The amounts of added styrene monomer and Au colloids were also critical to the formation of pure hybrid particles. When the amount of styrene monomer was increased by ∼1.5 times relative to Figure 1, some PS beads did not contain Au nanoparticles (Fig. S4) due to the homogeneous nucleation of PS. If the amount of monomer was decreased, the Au nanoparticles tended to aggregate. Taken together, we can conclude that one has to optimize the ratio of the monomer to Au colloids in order to eliminate the homogeneous nucleation of PS and agglomeration of Au colloids. In addition, both NaSS and ethanol could help PS oligomers to better nucleate on the surface of Au nanoparticles (supporting information).
This mechanism might only be applicable to Au colloids whose surfaces were stabilized by negative charges (we used Au colloids stabilized by citric and tannic acid from Ted Pella). When we tried to extend a similar protocol to Au colloids stabilized by poly(allylamine) (positive charges) and Au nanocages stabilized by poly(vinyl pyrrolidone) (PVP, neutral), we did not observe the hybrid structures (see Figs. S5 and S6). In these cases, it seems to be hard for the PS oligomers to displace the polymeric stabilizer already existing on the Au nanoparticles and in stead they tended to form plain PS beads through homogeneous nucleation.
While a systematic study is under way to achieve a complete understanding of the formation mechanism, we also examined the versatility of our method by using Au colloids of different sizes and different metal colloids. First, we successfully made Au-PS asymmetric particles by using home-made 20-nm Au colloids that were stabilized by citrate. As shown in Figure S7, we obtained the same asymmetric structure in a high yield. We next tried 40-nm Ag nanoparticles synthesized in the presence of citric acid as both a reductant and stabilizer. As shown in Figure S8, we could still obtain the asymmetric particles, in this case, consisting of Ag nanoparticles and PS beads.
In summary, we have prepared asymmetric, hybrid colloidal particles as a uniform sample using a precipitation polymerization method. Due to the simplicity of this process, we believe that this method can be applied to large-scale production. This procedure should be extendable to other different metal nanoparticles with appropriate surface charges, and the yield should not depend on the composition or size of the metal nanoparticles. Because of the uniform size but anisotropic composition, these hybrid particles are expected to find use as building blocks to fabricate new types of structures and devices.
Supplementary Material
Acknowledgements
This work was supported by a 2006 NIH Director’s Pioneer Award (5DP1OD00798-03). A.O. was partially supported by the foreign countries dispatch system of Hokkaido University Catalysis Research Center. E.C.C. acknowledges the support from the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, KRF-2007-357-D00070).
References
- (1).(a) Perro A, Reculusa S, Ravaine S, Bourgeat-Lami E, Duguet E. J. Mater. Chem. 2005;15:3745. [Google Scholar]; (b) Glotzer S, Solomon M. Nature Mater. 2007;6:557. doi: 10.1038/nmat1949. [DOI] [PubMed] [Google Scholar]; (c) Yang S, Kim S, Lim J, Yi G. J. Mater. Chem. 2008;18:2177. [Google Scholar]
- (2).(a) Takei H, Shimizu N. Langmuir. 1997;13:1865. [Google Scholar]; (b) Fujimoto K, Nakahama K, Shidara M, Kawaguchi H. Langmuir. 1999;15:4630. [Google Scholar]; (c) Love J, Gates B, Wolfe D, Paul K, Whitesides G. Nano Lett. 2002;2:891. [Google Scholar]; (d) Hugonnot E, Carles A, Delville M, Panizza P, Delville J. Langmuir. 2003;19:226. [Google Scholar]; (e) Cayre O, Paunov V, Velev O. Chem. Commun. 2003:2296. doi: 10.1039/b307296g. [DOI] [PubMed] [Google Scholar]; (f) Cayre O, Paunov V, Velev O. J. Mater. Chem. 2003;13:2445. [Google Scholar]; (g) Paunov V, Cayre O. Adv. Mater. 2004;16:788. [Google Scholar]; (h) Lu Y, Xiong H, Jiang X, Xia Y, Prentiss M, Whitesides G. J. Am. Chem. Soc. 2003;125:12724. doi: 10.1021/ja0373014. [DOI] [PubMed] [Google Scholar]; (i) Correa-Duarte M, Salgueirino-Maceira V, Rodriguez-Gonzalez B, Liz-Marzan L, Kosiorek A, Kandulski W, Giersig M. Adv. Mater. 2005;17:2014. [Google Scholar]; (j) Ohnuma A, Abe R, Shibayama T, Ohtani B. Chem. Commun. 2007:3491. doi: 10.1039/b705697d. [DOI] [PubMed] [Google Scholar]
- (3).(a) Yin Y, Lu Y, Xia Y. J. Am. Chem. Soc. 2001;123:771. doi: 10.1021/ja0031873. [DOI] [PubMed] [Google Scholar]; (b) Yin Y, Lu Y, Gates B, Xia Y. J. Am. Chem. Soc. 2001;123:8718. doi: 10.1021/ja011048v. [DOI] [PubMed] [Google Scholar]; (c) Xia Y, Yin Y, Lu Y, McLellan J. Adv. Funct. Mater. 2003;13:907. [Google Scholar]
- (4).(a) Gu H, Zheng R, Zhang X, Xu B. J. Am. Chem. Soc. 2004;126:5664. doi: 10.1021/ja0496423. [DOI] [PubMed] [Google Scholar]; (b) Akiva U, Margel S. Colloids Surf. A. 2005;253:9. [Google Scholar]; (c) Kim J, Larsen R, Weitz D. J. Am. Chem. Soc. 2006;128:14374. doi: 10.1021/ja065032m. [DOI] [PubMed] [Google Scholar]
- (5).(a) Reculusa S, Poncet-Legrand C, Ravaine S, Mingotaud C, Duguet E, Bourgeat-Lami E. Chem. Mater. 2002;14:2354. [Google Scholar]; (b) Yu H, Chen M, Rice P, Wang S, White R, Sun S. Nano Lett. 2005;5:379. doi: 10.1021/nl047955q. [DOI] [PubMed] [Google Scholar]; (c) Ge J, Hu Y, Zhang T, Yin Y. J. Am. Chem. Soc. 2007;129:8974. doi: 10.1021/ja0736461. [DOI] [PubMed] [Google Scholar]; (d) Camargo P, Xiong Y, Ji L, Zuo J, Xia Y. J. Am. Chem. Soc. 2007;129:15452. doi: 10.1021/ja077505a. [DOI] [PubMed] [Google Scholar]
- (6).(a) Roh K, Martin D, Lahann J. Nature Mater. 2005;4:759. doi: 10.1038/nmat1486. [DOI] [PubMed] [Google Scholar]; (b) Nisisako T, Torii T, Higuchi T. Chem. Eng. J. 2004;101:23. [Google Scholar]; (c) Nie Z, Li W, Seo M, Xu S, Kumacheva E. J. Am. Chem. Soc. 2006;128:9408. doi: 10.1021/ja060882n. [DOI] [PubMed] [Google Scholar]
- (7).Kamata K, Lu Y, Xia Y. J. Am. Chem. Soc. 2003;125:2384–2385. doi: 10.1021/ja0292849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


