Abstract
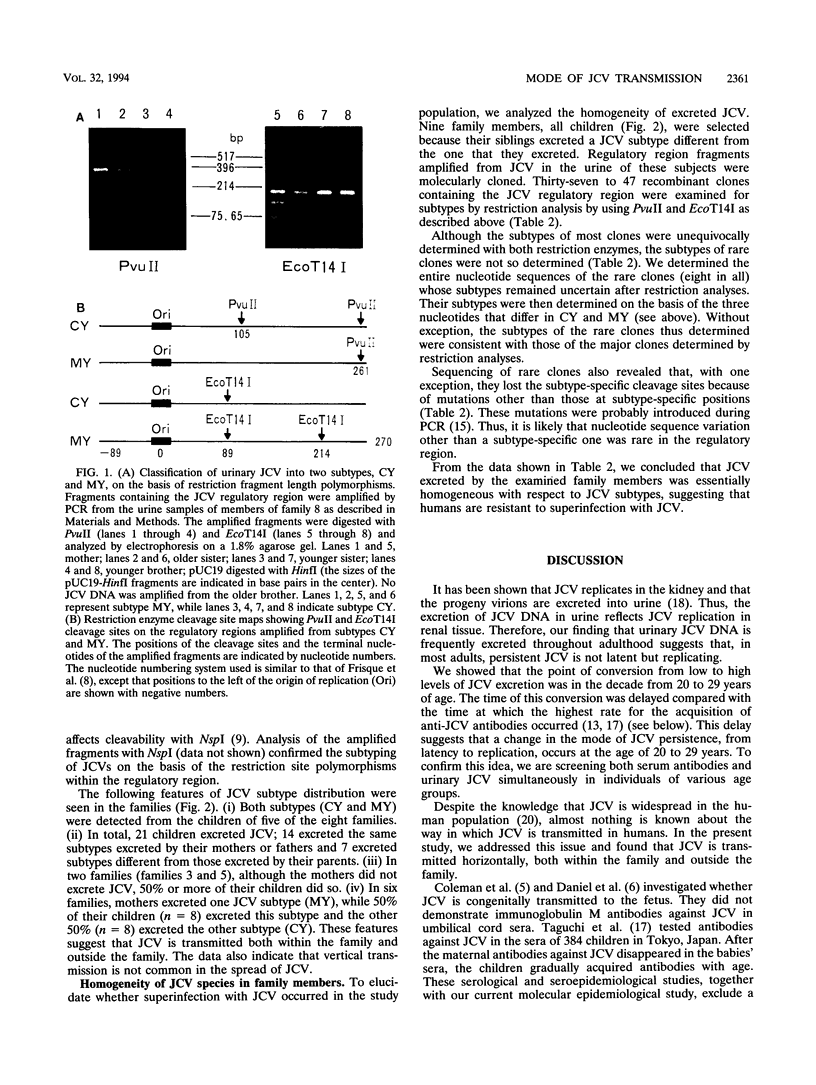
JC polyomavirus (JCV) is a ubiquitous symbiote in the human population, infecting children asymptomatically and then persisting in renal tissue. We reevaluated the urinary excretion of JCV in subjects in various age groups using PCR. The detection rate for urinary JCV DNA was 9 to 17% until the age of 20 years; this rate increased dramatically to about 46% at the ages of 20 to 29 years and then increased gradually with age. Therefore, it appears that in most people excretion of JCV begins at the age of 20 to 29 years, which is earlier than suggested previously. Next, we studied the way in which JCV is spread in the human population. We selected eight Japanese families in which both parents and children excreted JCV in their urine. Their JCV subtypes were determined by PCR amplification of a JCV DNA fragment; this was followed by restriction enzyme analysis. JCV species in all JCV-positive family members were classified into either of the two subtypes, subtypes CY and MY, which are prevalent in the Japanese population. The following features of JCV subtype distribution were seen in the families: (i) both subtypes were detected in children of five of the eight families, and (ii) of 21 children who excreted JCV, 14 children excreted the same subtypes excreted by their mothers or fathers, while the remainder (7 children) excreted subtypes different from those excreted by their parents. These features suggest that JCV is transmitted both within the family and outside the family. The data also indicate that vertical transmission is not common in the spread of JCV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur R. R., Shah K. V. Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. [PubMed] [Google Scholar]
- Ault G. S., Stoner G. L. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J Gen Virol. 1992 Oct;73(Pt 10):2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- Berger J. R., Kaszovitz B., Post M. J., Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987 Jul;107(1):78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- Chesters P. M., Heritage J., McCance D. J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983 Apr;147(4):676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Daniel R., Shah K., Madden D., Stagno S. Serological Investigation of the possibility of congenital transmission of papovavirus JC. Infect Immun. 1981 Jul;33(1):319–321. doi: 10.1128/iai.33.1.319-321.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries K. Progressive multifocal leucoencephalopathy: analysis of JC virus DNA from brain and kidney tissue. Virus Res. 1984 Jan;1(1):25–38. doi: 10.1016/0168-1702(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T., Kitamura T., Guo J., Taguchi F., Aso Y., Nagashima K., Yogo Y. Origin of JC polyomavirus variants associated with progressive multifocal leukoencephalopathy. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5062–5065. doi: 10.1073/pnas.90.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Aso Y., Kuniyoshi N., Hara K., Yogo Y. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J Infect Dis. 1990 Jun;161(6):1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Yogo Y., Kunitake T., Suzuki K., Tajima A., Kawabe K. Effect of immunosuppression on the urinary excretion of BK and JC polyomaviruses in renal allograft recipients. Int J Urol. 1994 Mar;1(1):28–32. doi: 10.1111/j.1442-2042.1994.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Kajioka J., Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26(11):1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Yogo Y., Kitamura T., Aso Y. Persistence of archetypal JC virus DNA in normal renal tissue derived from tumor-bearing patients. Virology. 1992 Feb;186(2):736–741. doi: 10.1016/0042-6822(92)90040-v. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Iida T., Taguchi F., Kitamura T., Aso Y. Typing of human polyomavirus JC virus on the basis of restriction fragment length polymorphisms. J Clin Microbiol. 1991 Oct;29(10):2130–2138. doi: 10.1128/jcm.29.10.2130-2138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Hara K., Iida T., Taguchi F., Tajima A., Kawabe K., Aso Y. Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol. 1991 May;65(5):2422–2428. doi: 10.1128/jvi.65.5.2422-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Ueki T., Aso Y., Hara K., Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990 Jun;64(6):3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]