Figure 2.
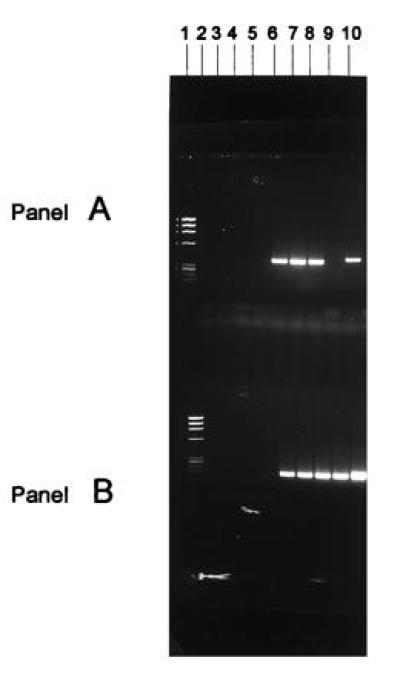
Solution DNA PCR analysis of vector MDR-1 positive cells. The cells from the suspension transduction protocol were incubated for an additional 24 hr in the presence of IL-3 and IL-6 growth factors in suspension culture after 6 hr of incubation with the vector before PCR assay. Cells that were subjected to the stromal or the suspension transduction procedures were cultured for an additional 24 hr after exposure to the retroviral vector in long-term culture on stromal monolayers before being collected for PCR assay. Cells were also obtained from the peripheral blood or bone marrow of patients at various times after transplant. DNA solution PCR analysis of vector MDR-1 sequences in the cells transduced with a MDR-1 retroviral vector. (A) DNA solution PCR analysis of vector MDR-1 sequences; (B) DNA solution PCR analysis of endogenous MDR-1 sequences. Lanes: 1, Phi-X174 molecular weight markers; 2, PCR buffer negative control; 3 and 4, marrow cells from ovarian cancer patient no. 9 unexposed and exposed to the MDR-1 vector suspension transduction method (both negative for the vector sequences); 5 and 6, nonadherent and adherent CD34+ cells from stromal cultures of breast cancer patient no. 5 not exposed to the MDR-1 vector (negative for MDR-1 vector sequences); 7–9, nonadherent hematopoietic cells, mixture of adherent hematopoietic cells and stromal cells, and stromal cells from cultures used for transduction with the MDR-1 vector stromal transduction method from breast cancer patient no. 5, respectively (all three samples positive for the vector MDR-1 sequences); 10, positive control consisting of cDNA from K562 cells known to contain the MDR-1 vector sequences.
