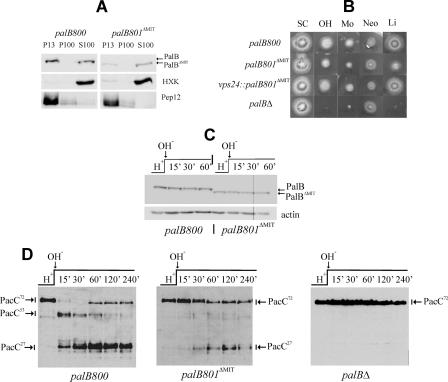
FIGURE 4.
Deletion of the PalB MIT domain impairs its association with membranes and the processing of PacC. A, MIT domain-mediated association of PalB with membrane fractions. Wild-type palB800 and mutant palB801ΔMIT protoplast lysates were fractionated into 13,000 × g insoluble (P13) and 100,000 × g insoluble (P100) and soluble (S100) fractions, which were analyzed by Western blotting. B, palB801ΔMIT results in weak loss-of-function in diagnostic plate tests of pH signaling (16, 74). palB800 is phenotypically wild-type (data not shown). In contrast, palB801ΔMIT confers some neomycin resistance (Neo), weakly impairs growth on alkaline pH (OH) and molybdate (Mo) plates, and decreases tolerance of LiCl (Li), indicating that deletion of the MIT domain results in weak loss-of-function; SC is synthetic complete medium without any addition. C, similar stability of PalB and PalBΔMIT in pH shift experiments. PalB was detected using α-HA antibody. Similar loading in the different lanes was determined using β-actin. palB800 and palB801ΔMIT cells cultured at acidic ambient pH were shifted to alkaline pH. Cell extracts were analyzed by Western blotting. D, palB801ΔMIT impairs PacC processing in pH shift experiments. Wild-type palB800 and null palBΔ controls are shown. The three forms of PacC are indicated.