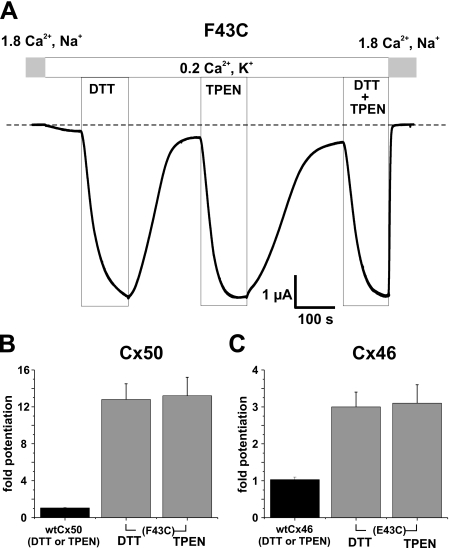
FIGURE 2.
Impaired function of F43C hemichannels is due to metal ion binding. A, shown are membrane currents in a F43C-expressing oocyte voltage clamped at -40 mV. Application of DTT (100 μm) or TPEN (10 μm), effective chelators of transition metal ions, caused large reversible increases in current amplitude. Potentiation of current caused by TPEN and DTT was similar in magnitude, and the effects of TPEN and DTT when applied together were not additive, indicating that impaired function is due to formation of metal bridges with the introduced cysteine. B, bar graph summarizing the effects of TPEN (n = 16) and DTT (n = 18) on F43C channels (left panel). Cx50 WT channels are not affected by TPEN or DTT (n = 12; solid bars). C, both TPEN and DTT also increased current magnitude in oocytes expressing Cx46 hemichannels in which the glutamic acid at position 43 was replaced with cysteine (i.e. E43C, right column). Because Cx46 activates only at positive voltages, current amplitudes were measured at the ends of 5-s depolarizing voltage steps to +40 mV. Mean current values after DTT (n = 6) and TPEN (n = 6) were again similar. Bars represent the mean ± S.E. of the fold-change caused by each chelator.