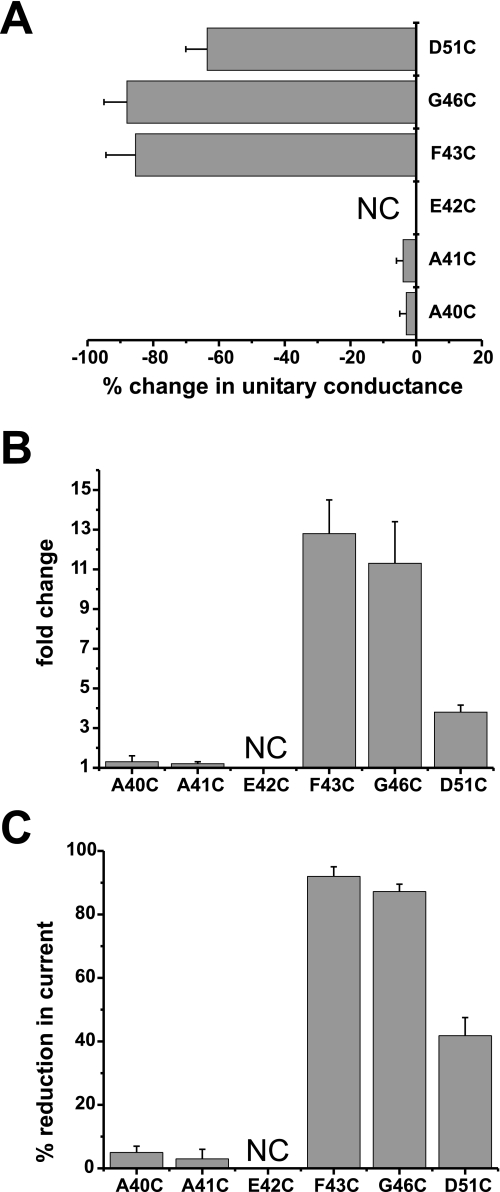
FIGURE 7.
Correspondence between residence in the pore and metal binding. A, SCAM using MTSEA-biotin shows three residues in E1 accessible to modification in single open hemichannels. Shown is a plot of the percentage change in unitary conductance for each residue after application of 100 μm MTSEA-biotin. The change in unitary conductance represents the mean percentage change in the slope conductance (measured at Vm = 0 from fitted open channel I-V relations) caused by MTS-biotin relative to unmodified cysteine-substituted channels for each mutant. Reductions in conductance were observed at three positions, F43C (n = 7), G46C (n = 7), and D51C (n = 6), but not at A40C (n = 5) or A41C (n = 5). B and C, potentiation by 100 μm DTT (B) and block by 1 μm Cd2+ (C) was robust for pore-lining residues F43C and G46C. D51C also was affected by DTT and Cd2+, but the magnitudes of changes were weaker in comparison. A40C and A41C hemichannels were not affected by DTT or Cd2+. Oocytes were voltage clamped to -40 mV. Bars represent the mean ± S.E. of the potentiation produced by DTT or block produced by Cd2+ (n ranged from 7 to 18).