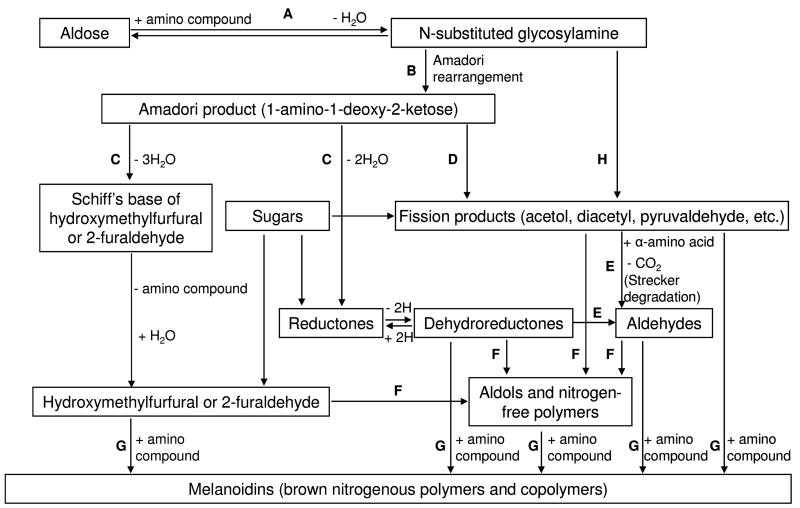
Figure 1. The Hodge Diagram.
A) The initial reaction between a reducing sugar and amino group forms an unstable Schiff base; B) The Schiff base slowly rearranges to form the Amadori product; C) Degradation of the Amadori product; D) Formation of reactive carbonyl and dicarbonyl compounds; E) Formation of Strecker aldehydes of amino acids and aminoketones; F) Aldol condensation of furfurals, reductones, and aldehydes produced in Steps C, D, and E without intervention of amino compounds; G) Reaction of furfurals, reductones, and aldehydes produced in Steps C, D, and E with amino compounds to form melanoidins; H) Free radical-mediated formation of carbonyl fission products from the reducing sugar (Namiki pathway).7, 8 Reproduced from Trends in Food Science and Technology, 1, Ames JM, Control of the Maillard reaction in food systems, pp. 150–154, 1990, with permission from Elsevier.