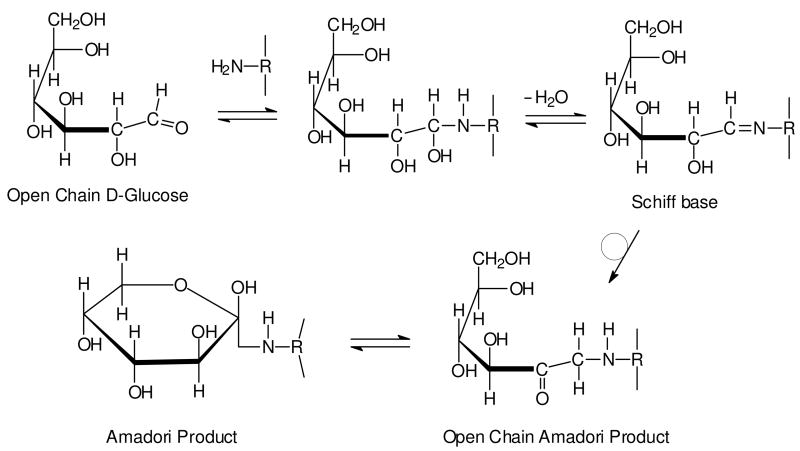
Figure 2. Reaction between glucose and amino group of protein to form the Amadori product.
Nucleophilic attack by a free amino group of protein on the aldehyde of glucose initially forms a carbinolamine, which subsequently dehydrates to a Schiff base. The Schiff base then undergoes a slow rearrangement to form the Amadori product. While only a single cyclic isoform of the Amadori product is shown, it is important to note that it exists as a mixture of several isoforms.