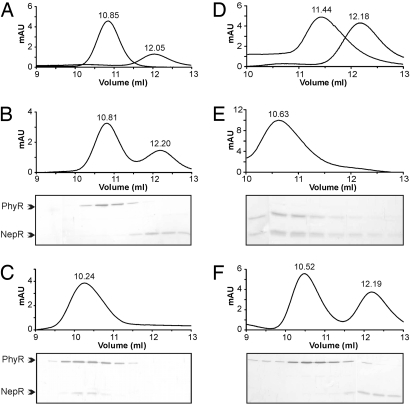
Fig. 2.
Phosphorylated PhyR directly interacts with NepR via its N-terminal ECF sigma factor-like domain. Protein complexes were resolved on a Superdex 75 10/300 column (flow rate, 0.5 mL/min). The elution volume is indicated above the absorption peak. Fractions were subsequently analyzed on a 15% SDS-polyacrylamide gel and silver stained. (A) The elution profile of purified PhyR and NepR (each 3 μM) passed separately over the column. (B) No interaction of PhyR and NepR was observed when incubated in equimolar amounts (each 3 μM) in the absence of acetyl phosphate. (C) Upon the addition of acetyl phosphate to the binding buffer (25 mM), the formation of a heterodimeric complex of phosphorylated PhyR and NepR was observed. (D) Separate gel filtration of the purified N-terminal ECF sigma factor-like domain of PhyR and NepR. (E) The ECF domain of PhyR and NepR showed a direct interaction, even in the absence of acetyl phosphate. (F) A PhyR D190A mutant could not form a complex with NepR in the presence of 25 mM acetyl phosphate.