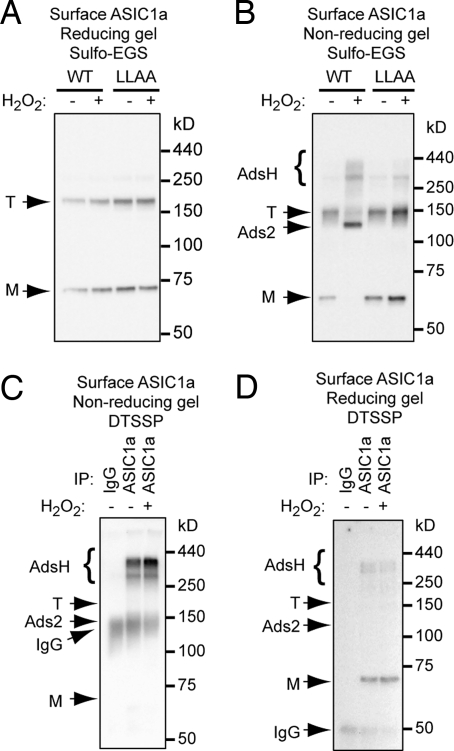
Fig. 6.
Inter-subunit disulfide bonds may form higher-order ASIC1a complexes. WT ASIC1a and LLAA mutants were expressed in CHO cells and cross-linked with 0.7 mM sulfo-EGS at 4 °C and then biotinylated. Surface fraction was analyzed by reducing (A) or non-reducing (B) Western blots. On a reducing gel, WT and LLAA mutant showed similar levels of trimer (T) and monomer (M). On a non-reducing gel, H2O2 decreased band T and increased Ads2 and AdsH of WT ASIC1a. In contrast, H2O2 had no effect on LLAA mutant. Blots are representative from 3 experiments. (C and D) Surface proteins were cross-linked with a membrane-impermeable reducible cross-linker, DTSSP. ASIC1a was immunoprecipitated with anti-HA antibody, followed by blotting for biotin. Under non-reducing conditions (C), most of the protein ran at the band H position. Under reducing conditions (D), most of the biotinylated protein migrated as the ASIC1a monomer. The position of IgG is also indicated.