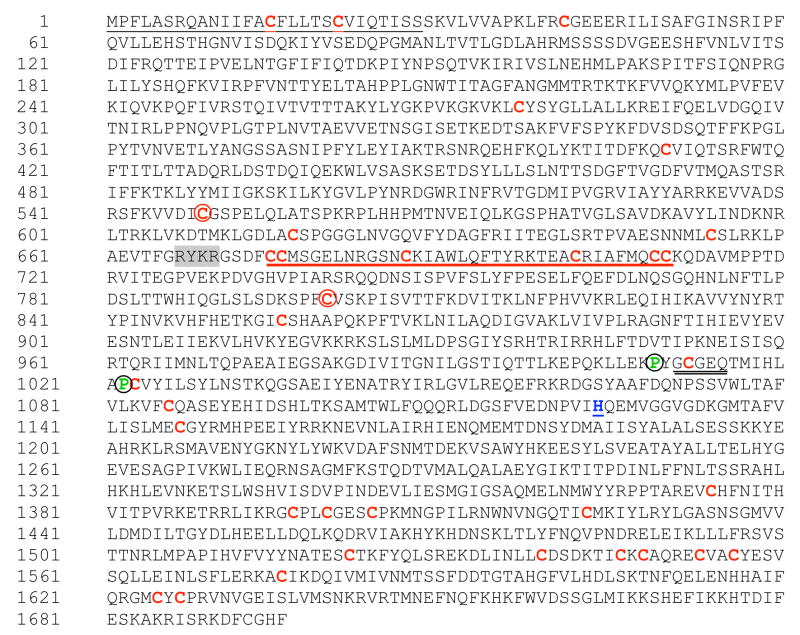
Fig. 1.
The deduced amino-acid sequence of Es-C3. The ORF predicts a protein of 1696 amino acids with characteristic domains and motifs of other known complement C3 components. Highlighted are: the 28 amino-acid peptide of the secretion signal (black underline, N-terminus); the conserved cysteine residues (red), including the two cys (squared) that are thought to be part of the disulfide bond linking the α and β chains in the mature and activated C3b molecule; the ANATO domain (red underline); the thioester domain (double black underline); the neighboring proline residues (green, circled), known to be important for the thioester domain stability. The catalytic histidine residue (blue, underline); and, the potential processing site for the cleavage of the alpha and beta subunits (gray background).