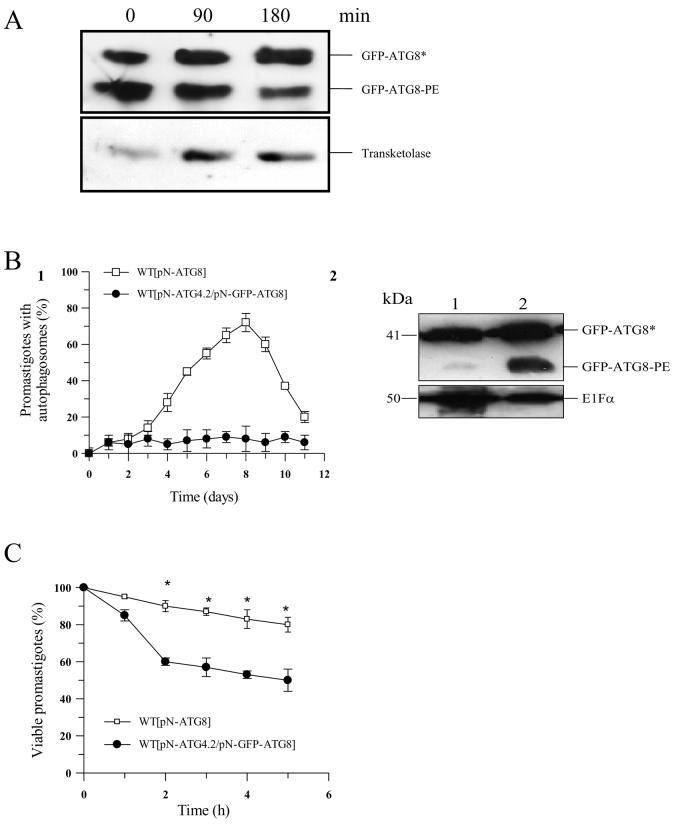
Figure 5. A role for ATG4.2 in processing lipidated ATG8 in L. major.
A. Western blot analysis using α-GFP antibody on Δatg4.2 [pN-ATG8] promastigote cell extracts (5-8 × 106 cells ml−1) show an abundance of lipidated ATG8 (labelled GFP-ATG8-PE) (lane 0). Incubation of the cell extract with recombinant ATG4.2 for 90 and 180 min at 30°C resulted in a decrease in GFP-ATG8-PE (lanes 2 and 3). Transketolase was used as an internal loading control.
B1. WT[pN-ATG8] (open squares) and WT[pN-ATG4.2/ pN-GFP-ATG8] (closed circles) promastigotes were compared for the occurrence of putative autophagosomes during growth in vitro in normal medium. Data are means ± SD from 3 independent experiments.
B2. Western blot analysis of extracts from early stationary phase WT[pN-ATG4.2/pN-GFP-ATG8] (lane 1) and WT[pN-GFP-ATG8] (lane 2) promastigotes separated by SDS-PAGE containing 6 M urea and detected with α-GFP antibody. Cleaved ATG8 (GFP-ATG8*) and lipidated ATG8 (GFP-ATG8-PE) are indicated. EF1α was used as an internal loading control.
C. Sensitivity to starvation of WT[pN-ATG8] (open squares) and WT[pN-ATG4.2/pN-GFPATG8] (closed circles) L. major promastigotes. Cells were incubated in PBS and their viability assessed by the MTT assay. Data are means ± SD from four replicates. *: data for WT[pN-ATG4.2/pN-GFP-ATG8] and WT[pN-GFP-ATG8] promastigotes differed significantly (P < 0.05).