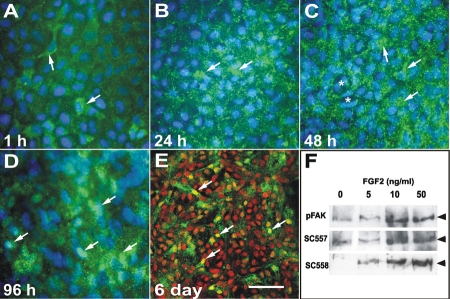
Figure 4.
Effect of FGF2 on FAK expression and localization. Immunolocalization of phosphoY397-FAK (green) in untrimmed explants treated with FGF2 for 1 h (A), 24 h (B), 48 h (C), 96 h (D), and 6 days (E) and counter-stained with Hoechst (blue) or propidium iodide (red) to label nuclei. A: After 1 h of culture occasional epithelial cells (arrows) showed distinct punctate cytoplasmic reactivity for phospho-FAK. B: Reactivity increased after 24 h and almost all cells in the explant showed distinct punctate reactivity suggestive of adhesion junctions. C: A similar pattern of expression was observed at 48 h. Cells that were starting elongate (arrows) showed strong reactivity, whereas cells that had undergone division (asterisk) showed less distinct staining. D: By 96 h the elongating fiber cells showed distinct punctate FAK reactivity, some of which appeared to be present in the nucleus (arrows). As differentiating explants are multilayered we used confocal microscopy to confirm the nuclear localization. E: Confocal microscopy on explants cultured for 6 days in the presence of FGF2 showed distinct punctate phospho-FAK reactivity (green) in the cytoplasm of elongated fiber cells. Co-staining with propidium iodide, which labels nuclei red clearly showed nuclear staining (yellow) for phospho-FAK (arrows). F: Western blots of explants treated with FGF2 (0, 5, 10, or 50 ng/ml) for 5 days and probed with phosphoY397-FAK (pFAK), NH2-terminal (SC-557) and COOH-terminal (SC-558) antibodies. In control explants (0 ng/ml FGF2) low levels of FAK were detected. A slight increase in FAK expression and phosphorylation was detected with a proliferative dose of FGF2 (5 ng/ml). At doses of FGF2 that induce cell migration (10 ng/ml) and differentiation (50 ng/ml) there was marked increase FAK expression and phosphorylation. The scale bar in A-D indicate 15 μm and in E, 30 μm.