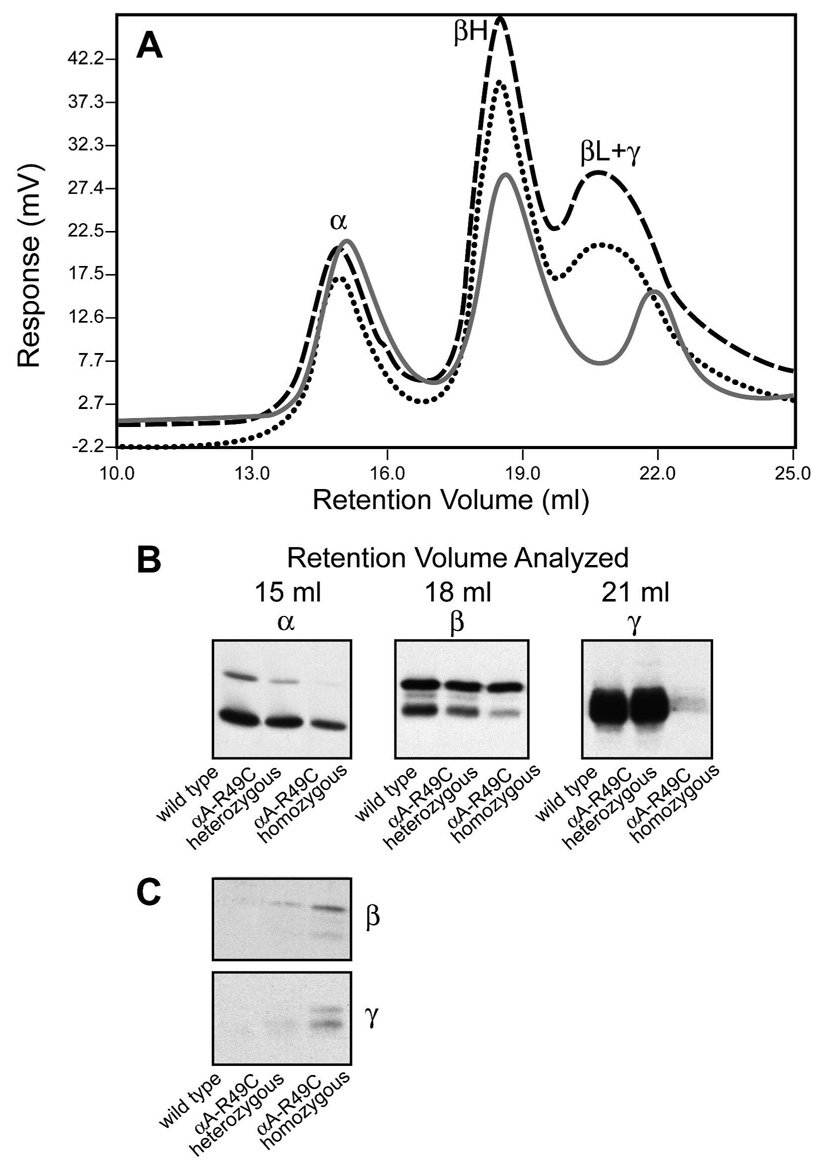
Figure 4. UV absorbance and immunoblot analysis of lens protein fractions from wild type and αA-R49C mutant lenses.
Wild type lenses (dashed line); αA-R49C heterozygous lenses (dotted line); αA-R49C homozygous lenses (solid gray line). (A) UV absorbance at 280 nm; (B) Immunoblots represent the peak fractions of α-crystallin, βH-crystallin and βL- +γ-crystallin obtained from chromatographic separation of wild type, αA-R49C heterozygous and αA-R49C homozygous lens water-soluble proteins, respectively. Immunoblot analysis of the chromatography peak fractions with antibodies to αA-crystallin, total β-crystallin and total γ-crystallin was performed. Note the distinct loss of immunoreactivity to γ-crystallin antibody with peak 4 (γ-crystallin) in the αA-R49C homozygous lens proteins. Note also that the concentration of the water-soluble α-crystallin fraction (αA + αB) was the highest in the wild type, and decreased gradually in the αA-R49C heterozygous and homozygous lenses. (C) Immunoblot analysis of the α-crystallin fraction of wild type, αA-R49C heterozygous and αA-R49C homozygous lenses with antibodies to β- and γ-crystallin. Note the gradual increase in β- and γ-crystallin immunoreactivity in the heterozygous and homozygous α-crystallin fractions. Note also that β- and γ-crystallin immunoreactivity was absent in the wild type α-crystallin fraction.