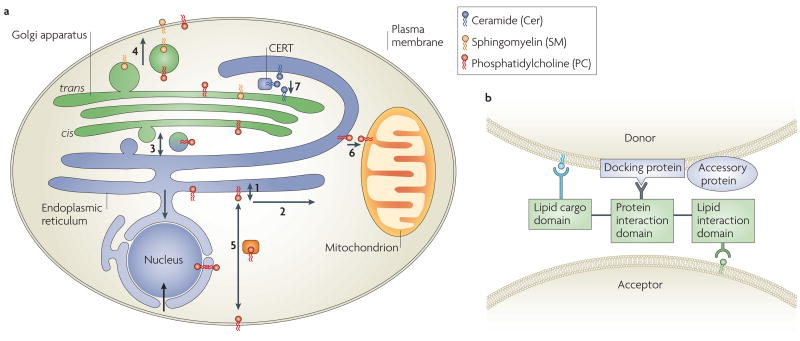
Figure 4. Emerging models for lipid transport.
a Phosphatidylcholine (PtdCho (PC); red) is synthesized on the cytosolic endoplasmic reticulum (ER) surface (1) and freely flips across the ER membrane. On both sides it diffuses laterally in any membrane (2). PtdCho travels through vesicles to the Golgi (3), the plasma membrane (4) and endosomes (not shown). It is transported by transfer proteins between the cytosolic surfaces of organelles (5), maybe through contact sites (6 and 7). The ceramide (Cer) transfer protein CERT transports Cer from the ER to the Golgi (7) for lumenal sphingomyelin (SM) synthesis. SM cannot flip and travels in the vesicle lumen (5). Plasma-membrane enrichment of SM and cholesterol predicts their concentration at anterograde budding sites. Adapted from REF. 8. A model for non-vesicular lipid transport predicts carrier protein interaction with a donor membrane protein, promoting ligation of the cargo lipid (green). Cargo engagement facilitates carrier dissociation from the donor and diffusion to the acceptor membrane, which is recognized by distinct phosphoinositides. Binding between the carrier and the acceptor membrane induces cargo release, which is predicted to reduce the affinity of the carrier for the acceptor membrane and enable re-initiation of the cycle. Accessory proteins on donor and/or acceptor membranes may enhance the association and dissociation of carrier proteins. They may also facilitate the formation of contact sites, which restricts the diffusion path of the lipid carrier. The model provides the necessary elements of directionality and specificity to produce net lipid transfer.