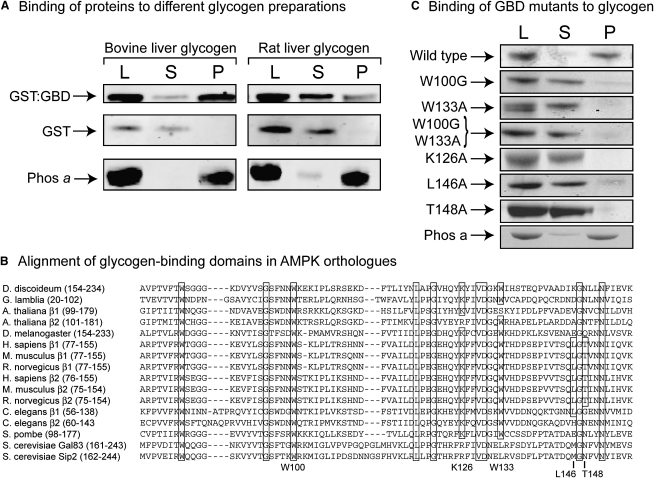
Figure 1.
Studies on Binding of Glycogen to Bacterially Expressed β1-GBD
(A) Binding of GST:GBD fusion, free GST, and phosphorylase a to glycogen. Samples of each protein were incubated with bovine or rat liver glycogen bound to ConA-Sepharose, the Sepharose beads were recovered by centrifugation, and samples of the load (L), supernatant (S), and pellet (P, resuspended in the original volume) were analyzed by SDS-PAGE.
(B) Alignment of GBD sequences from various eukaryotes made using ALIGNX. Residues identical in all species are boxed, as are conserved residues in mammalian species directly involved in carbohydrate binding; the latter are identified at the bottom (rat β1 numbering).
(C) Binding to glycogen of GST:GBD fusions (wild-type rat β1 or the point mutations shown). The binding assay was as in (A) using bovine liver glycogen, and binding of phosphorylase a was analyzed as a positive control (bottom panel).