Abstract
Former vascular endothelial growth factor (VEGF) - head and neck squamous cell carcinoma (HNSCC) studies have focused on VEGF’s contributions toward tumor-associated angiogenesis. Previously, we have shown that HNSCC cells produce high levels of VEGF. We therefore hypothesized that VEGF serves a biphasic role i.e. proangiogenic and protumorigenic in HNSCC pathogenesis. Western blots confirmed the presence of VEGF’s primary mitogenic receptors, VEGFR-2/KDR and VEGFR-1/Flt-1 in cultured HNSCC cells. Subsequent studies evaluated VEGF’s effects on HNSCC intracellular signaling, mitogenesis, invasive capacities and matrix metalloproteinases (MMPs) activities. Introduction of hrVEGF165 initiated ROS-mediated intracellular signaling, resulting in kinase activation and phosphorylation of KDR and Erk1/2. As high endogenous VEGF production rendered HNSCC cells refractory to exogenous VEGF’s mitogenic effects, siRNA was employed, inhibiting endogenous VEGF production for up to 96h. Relative to transfection vector matched controls, siRNA treated HNSCC cells showed a significant decrease in proliferation at both 30nM and 50nM siRNA doses. Addition of exogenous hrVEGF165 (30ng/ml and 50ng/ml) to siRNA-silenced HNSCC cells resulted in dose-dependent increases in cell proliferation. Cell invasion assays showed VEGF is a potent HNSCC chemoattractant and demonstrated that VEGF pretreatment enhanced invasiveness of HNSCC cells. Conditioned media from VEGF challenged HNSCC cells showed a moderate increase in gelatinase activity. Our results demonstrate, for the first time, that HNSCC cells are both targets and effectors for VEGF. These data introduce the prospect that VEGF targeted therapy has the potential to fulfill both anti-angiogenic and anti-tumorigenic functions.
Keywords: VEGF, KDR, Flt-1, Head and neck squamous cell carcinoma, intracellular signaling
Vascular endothelial growth factor (VEGF) is involved with every stage of vascular development promoting survival, migration, specialization and proliferation of endothelial cells [Ferrara et al., 2003]. The resulting formation of new blood vessels and vascular network (neovascularization) is essential for both normal growth and development and also for sustaining the progression, invasion and metastasis of solid tumors [Folkman, 1992]. The transition of tumor cells to an angiogenic phenotype, referred to as the “angiogenic switch”, contributes to the disruption of balance between angiogenic promoters and inhibitors [Hanahan and Folkman, 1996]. Experimental and clinical reports confirm that VEGF plays a central role in regulating angiogenesis and vasculogenesis in solid tumors and is tightly associated with the angiogenic switch [Ribatti et al., 2007; Naumov et al., 2006]. This transition to a pro-angiogenic phenotype has been demonstrated to be crucial in the progression of oropharyngeal epithelial dysplasia to invasive head and neck squamous cell carcinoma (HNSCC) [Hasina and Lingen, 2001; Johnstone and Logan, 2006].
HNSCC is the sixth most common human cancer worldwide with a long-term survival rate of less than 50% [Chin et al., 2006]. Radical surgery remains the primary treatment modality for carcinomas of the head and neck [Gourin et al., 2005]. The economic, physiological and psychosocial impacts of this treatment modality are substantial, especially in cases where post-surgical disfigurement and/or disability result [Chin et al., 2006; Gourin et al., 2005]. Furthermore, many patients who have had an HNSCC removed encounter tumor recurrence and/or develop a second primary HNSCC [Chin et al., 2006; Gourin et al., 2005]. Current research efforts are accordingly focused towards the discovery of adjuvant therapies to suppress progression of premalignant disease and/or inhibit HNSCC recurrence or development of second primaries [St John et al., 2006]. Growth factors and their receptors are leading targets for such tumor-directed therapies [Fujita et al., 2007; Zhang et al., 2007; Sano et al., 2007]. A phase II clinical trial led by Fury et al. [2007] showed that treatment targeting VEGFR-2 decreased tumor vascularity in 5 of 7 HNSCC patients.
As a central mediator of angiogenesis, VEGF expression is increased in many cancers [Ferrara, 2005]. A previous study from our lab demonstrated that cultured HNSCC cells produced VEGF levels were appreciably higher than other human cancers and normal keratinocytes [Rodrigo et al., 2006]. Related studies, which quantified VEGF expression in HNSCC tumors, failed to show an association between VEGF secretion and microvessel density [Moriyama et al., 1997; Artese et al., 2001]. Collectively, these data suggest that HNSCC-produced VEGF may fulfill other functions in addition to angiogenic induction [Kyzas et al., 2005]. Like most cytokines, VEGF isoforms elicit intracellular effects by binding to and activating their analogous receptors [Olsson et al., 2006]. At least three receptor tyrosine kinases (RTKs) have been identified for VEGF; among which, VEGFR-2 (or kinase insert domain-containing receptor, KDR), is considered the dominant mediator of mitogenesis in endothelial cells [Kanno et al.,2000]. VEGF receptors were once thought to be expressed exclusively on endothelial origin cells [Breier et al., 1995; Millauer et al., 1993]. Recent studies, however, discovered that human epithelial lineage cancer cells including head and neck cancers also express VEGFR-1 and/or VEGFR-2 [Neuchrist et al., 2001; Lalla et al., 2003; Stewart et al., 2003]. Clinical data have shown that high VEGF levels in HNSCC tumors were positively correlated with both KDR expression and HNSCC tumor progression [Kyzas et al., 2005].
VEGFR-1/Flt-1 was found to be associated with mediation of the “epithelial to mesenchymal transition” (EMT) in human pancreatic and colon carcinomas [Yang et al., 2006; Bates et al., 2003]. Recent studies also suggest a phenotypic plasticity, which enhances invasiveness and ultimately metastasis, exists in some carcinomas. [Thiery et al., 1999; Christiansen et al., 2006; Gotzmann et al., 2004]. Expression of genes associated with EMT has been shown to be one of the most significant molecular characteristics demonstrated by high risk HNSCC tumors [Chung et al., 2006]. Studies from our lab, which showed the angiostatic agent, endostatin, significantly reduced HNSCC cell migration and invasion, implied that endothelial-like mesenchymal phenotypic properties are retained in cultured HNSCC cells [Wilson et al., 2003].
Based on these clinical and experimental data, this study investigated the hypothesis that HNSCC generated VEGF fulfills a direct pro-tumorigenic role via autocrine-paracrine HNSCC cellular effects. Our results, which show VEGF elicits HNSCC intracellular signaling, stimulates proliferation and induces invasion in HNSCC cells, support this premise.
MATERIALS AND METHODS
Cell Culture
Three human head and neck squamous cell carcinoma cell lines (SCC4, SCC9, and SCC15), derived from squamous cell carcinomas of the tongue, were obtained from the American Type Cell Culture (ATCC, Manassas, VA). For expansion, cells were cultured in DMEM/F-12 medium supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY) at 37°C, 5% CO2. Human umbilical vein endothelial cells (HUVECp, Cascade Biologics, Portland, OR) were cultured in Medium 200 supplemented with LSGS (Cascade Biologics, Portland, OR) at 37°C, 5% CO2. In order to investigate VEGF-specific cellular effects, serum free medium was used in selected experiments as described.
Western Blot Analysis
HUVECs or HNSCC cells which had undergone 24 h sera deprivation, were incubated with (20 min incubation) or without human recombinant VEGF165 (hrVEGF165, R&D Systems, Minneapolis, MN) in fresh sera free medium. The cell monolayers were then washed with ice cold PBS and lysed with M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) with 1X protease inhibitor cocktail or 1X phosphatase inhibitor cocktail (Pierce, Rockford, IL). Total protein concentrations were detected by Bio-Rad protein assay (Hercules, CA). Samples containing equivalent amounts of protein were then separated by SDS-PAGE on 6% or 10% acrylaminde/bis gels, and transferred to Hybond-P PVDF membranes (Amersham, Piscataway, NJ). Electrophoresis duration was prolonged in experiments investigating KDR isoforms to achieve a distinctive separation at high molecular range. Immunoblotting was conducted using standard methods. Proteins were visualized using the enhanced chemiluminescence (ECL) system (Amersham, Piscataway, NJ) followed by exposure to CL-Xposure films (Kodak, Rochester, NY). The antibodies and working dilutions were as follows: Flk-1/KDR mouse monoclonal antibody (1:200 dilution, Santa Cruz, CA), Flt-1 rabbit polyclonal antibody (1:200, Abcam), phosphorylated Flk-1/KDR (Tyr951) rabbit polyclonal antibody (1:200, Santa Cruz, CA), Erk1/2 (p44/42) mouse monoclonal antibody(1:2000, Cell Signaling Tec., Boston, MA), and phosphor-Erk1/2 (phospho-p44/42) polyclonal rabbit antibody (1:1000, Cell Signaling Tec., Boston, MA). Kodak 1D3 image analysis software (Kodak, Rochester, NY) was employed to perform densitometry analyses. Data were normalized relative to protein levels of β-actin, which was probed by a mouse monoclonal antibody (1:1000, Santa Cruz, CA).
Detection of Intracellular Reactive Oxygen Species (ROS)
HNSCC cells (1×104/chamber) were seeded on Lab-Tek chamber slides (Nalge Nunc Int. Naperville, IL) in serum free DMEM/F12 medium (base medium) and were incubated at 37°C, 5% CO2 for 24 h. The conditioned media were removed, followed by addition of fresh base medium with PBS (negative control), 0.1mM H2O2 (positive control) or two doses of hrVEGF165 (30ng/ml and 50ng/ml). Chamber slides were incubated at 37°C for 15 min. Cells were then treated with 10µM of reactive oxygen species detecting probe CM-H2 DCFDA (Molecular Probes, Eugene, OR) and incubated for an additional 5 min. Generation of intracellular reactive oxygen species was observed with an Olympus fluorescence microscope.
SiRNA Transfection
Pre-designed siRNA against VEGF and FAM™-Labeled GAPDH siRNA were purchased from Ambion Inc. (Austin, TX). The sense sequence for VEGF siRNA is: 5’-GGAGGAGGGCAGAAUCAUCtt-3’ and the antisense sequence is: 5’- GAUGAUUCUGCCCUCCUCCtt-3’. The siRNA was mixed with the transfection agent SiPORT NeoFX (Ambion, Austin, TX) 10 min before pre-coating the culture plates at a final concentration of 30nM or 50nM. HNSCC cells were then seeded at 3×104 cells/well in the siRNA pre-coated 24-well plates. 30nM GAPDH siRNA was used as the positive control, and 2µl/well of the transfection agent NeoFX alone was used as the negative control. Transfected cells were incubated at 37°C, 5% CO2, for 24 h. The conditioned media of each well was then collected for VEGF ELISA analyses or replaced by serum free DMEM/F-12 base medium with or without VEGF for the cell proliferation assay, according to the experimental plan.
Enzyme-Linked ImmunoSorbent Assay
Samples of the conditioned medium obtained at 24, 48, 72, and 96 h after siRNA transfection were analyzed by ELISA (Human VEGF DuoSet ELISA Development Kit, R&D Systems, Minneapolis, MN) to determine the concentration of VEGF protein secreted by HUVEC and HNSCC cells. Results are expressed as pg/104 cells with cell numbers determined as described in the following section.
VEGF Induced Cell Proliferation
Twenty four hours after siRNA transfection, HNSCC cells were stimulated with 0 (control), 30ng/ml, or 50ng/ml hrVEGF165 in fresh serum free base medium. Every 24 h, conditioned media were removed, followed by addition of fresh base media with the appropriate treatment to the 24-well plates. Each experimental group had at least 4 replicate wells. HUVECs were used as positive controls to validate hrVEGF165’s mitogenic activity. Cell numbers were determined using the MTT (methylthiazoyltetrazolium chloride) assay (CellTiter Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI). Cell harvest time points were: 0, 24, 48, and 72 h after hrVEGF165 stimulation. A matched cell line specific standard curve was run concurrently with each assay.
Cell Invasion Assay
Following 24 h sera deprivation, conditioned media were removed and HNSCC cells were incubated with fresh serum free base medium (negative control), 10% FBS supplemented base medium (positive control), or pretreated with 100ng/ml hrVEGF165 for an additional 24 h prior to the cell invasion assay (InnoCyte cell invasion kit, Calbiochem, San Diego, CA). Cells (4 ×104/well) were then seeded in the upper chambers with fresh serum free base medium. 10% FBS or 100ng/ml hrVEGF165 were added to selected lower chambers as chemoattractants. The invasion plates were then incubated at 37°C, 5% CO2 for 48 h. A standard curve consisting of cell line matched cells was run concurrently with each assay for determination of invaded cells.
MMP2 and MMP9 Zymography
Twenty four hours sera deprived HNSCC cells (1×107) were grown in fresh sera free base medium with or without 100ng/ml hrVEGF165 for an additional 24 h. Conditioned media were then collected and clarified by cold centrifugation at 1000g for 10 min. Sample media with or without 2 h pre-incubation of the pro-MMP activator paminophenylmercuric acetate (APMA, 1mM, Calbiochem, San Diego, CA) were separated by a 7.5% non-reducing SDS-PAGE gel copolymerized with 0.1% gelatin. The gelatinolytic activities were analyzed as described previously [Pei et al., 2006]. Densitometry analysis was performed by Kodak 1D3 image analysis software.
Statistical Analysis
siRNA and cell invasion data were analyzed by one way ANOVA followed by Tukey-Kramer multiple comparisons test. Analysis of the cell proliferation data were conducted using the unpaired t test. Graphpad InStat 3 (Graphpad Software Inc., San Diego, CA) software was employed for the statistical analyses. The level of significance was established at p<0.05.
RESULTS
Cultured HNSCC cells retain VEGFR-2/KDR and VEGFR-1/Flt-1
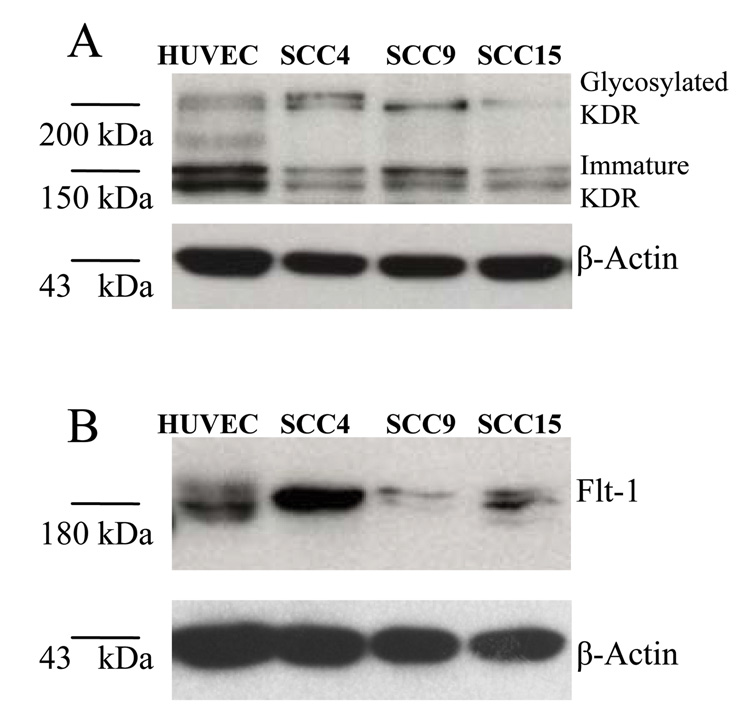
The primary translational product of KDR is a 150 kDa immature protein. During post-translational processing, KDR protein is rapidly glycosylated to a 200 kDa intermediate form. The functional mature KDR is a fully glycosylated cross-membrane protein with a molecular weight of 230 kDa [Takahashi and Shibuya, 1997]. Western blots conducted on three HNSCC cell lines demonstrated the presence of KDR (both immature and intermediate forms) and Flt-1. Relative to HNSCC cells, HUVECs were found to contain higher levels of the immature KDR (150kDa) protein. One HNSCC cell line (SCC4) demonstrated higher levels of Flt-1 compared to HUVECs (Figs. 1A and 1B).
Fig. 1.
Cultured HNSCC cells retain possession of VEGFR-2/KDR and VEGFR-1/Flt-1. A and B: Three HNSCC cell lines, SCC4, SCC9, and SCC15, were screened for the cytoplasmic production of KDR and Flt-1 proteins by Western Blot. HUVECs were included as the positive control.
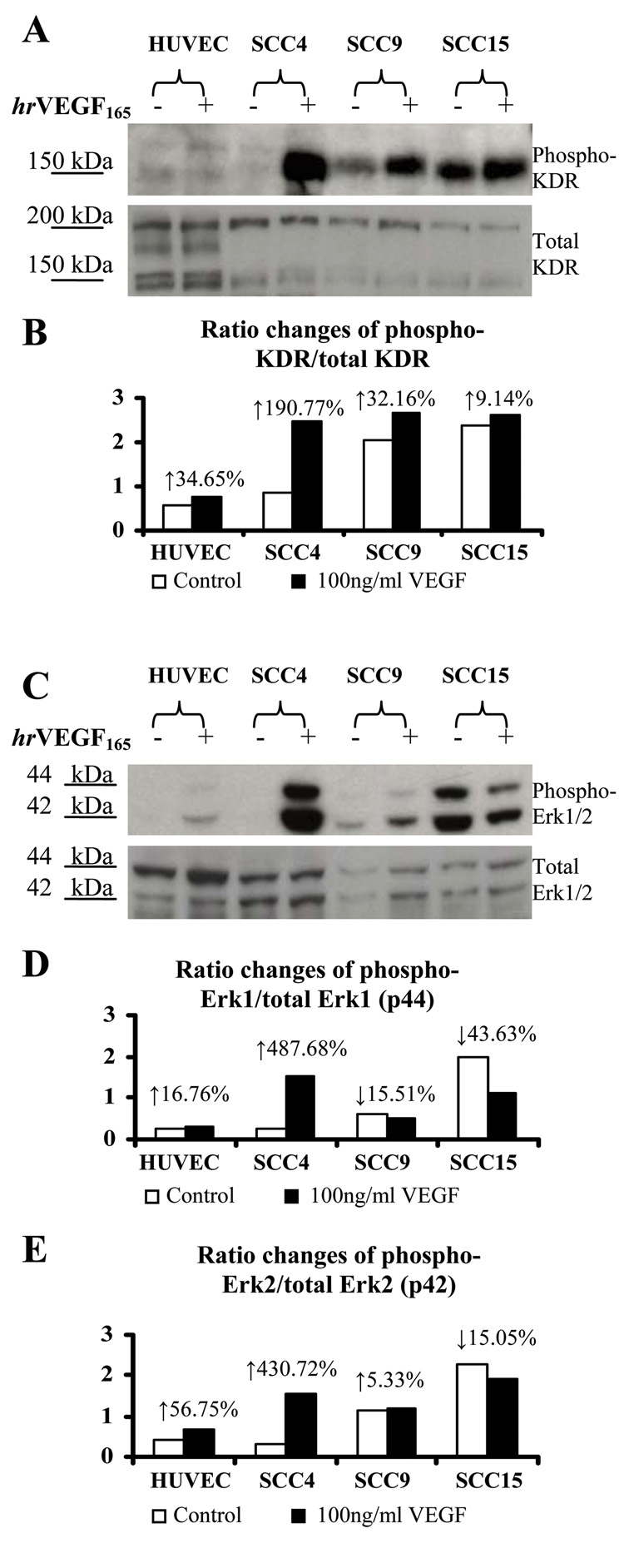
hrVEGF165 increases phosphorylated levels of KDR and Erk1/2 in HNSCC cells
Introduction of hrVEGF165 (100ng/ml) to HNSCC cells increased phosphorylated levels of KDR relative to the PBS matched negative controls in every cell line evaluated (Fig. 2A). Increased phosphorylated Erk1/2 levels were also observed in two of three HNSCC cell lines (SCC4 and SCC9) in response to VEGF stimulation (Fig. 2C). In contrast, SCC15 cells showed high baseline levels of phosporylated Erk1/2 and demonstrated a decrease in Erk1/2 phosphorylation following VEGF introduction (Fig. 2C). Densitometry analyses revealed cell line specific differences in phosphorylation ratios of KDR and Erk1/2 following VEGF challenge (Figs. 2B, 2D and 2E). Of all cell lines evaluated, SCC4 was unique with regard to the high extent of VEGF-mediated kinase activation.
Fig. 2.
VEGF induced phosphorylation of KDR and Erk1/2 in cultured HNSCC cells. A and C: HUVECs and 24h serum deprived HNSCC cells were challenged with PBS (negative control) or 100ng/ml VEGF for 20min in serum free media. Western Blot analyses were then conducted to determine phosphorylated and total levels of KDR and Erk1/2. hrVEGF165 activated intracellular kinases, resulting in increased phosphorylated ratios of KDR and Erk1/2. Densitometry analyses results were demonstrated in panels B, D and E, respectively.
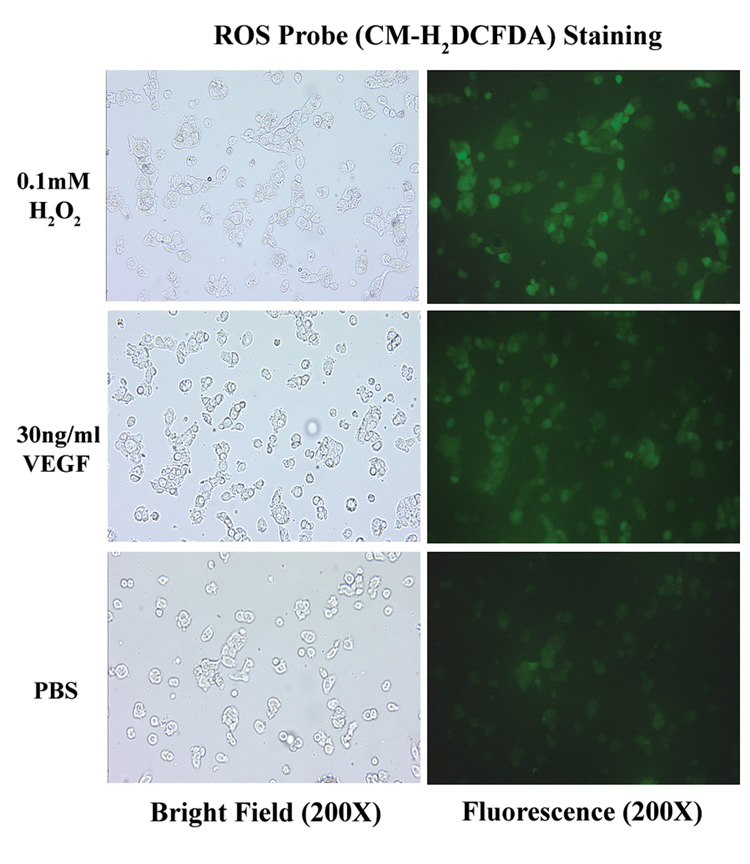
Addition of hrVEGF165 initiates intracellular reactive oxygen species (ROS) generation in HNSCC cells
Recent studies show that VEGF induces rapid increase in the intracellular generation of reactive oxygen species via activation of VEGFR-2/KDR in endothelial cells [Colavitti et al., 2002]. We have asked whether VEGF has a similar effect on epithelial origin HNSCC cells. Our data revealed that VEGF induced comparable fluorescence intensities as the positive control H2O2 in all HNSCC cell lines. (Fig. 3)
Fig. 3.
Introduction of hrVEGF165 initiated release of intracellular reactive oxygen species. HNSCC cells were seeded on chamber slides and incubated at 37°C, 5% CO2 for 24 h in serum-free medium. 0.2mM H2O2 (positive control) , 30ng/ml hrVEGF165 or equal volume of PBS (negative control) were then added to the chambers and incubated for 15 min, followed by 5 min staining of 10µM CM-H2 DCFDA. Increases in fluorescence intensities reflect intracellular probe oxidation.
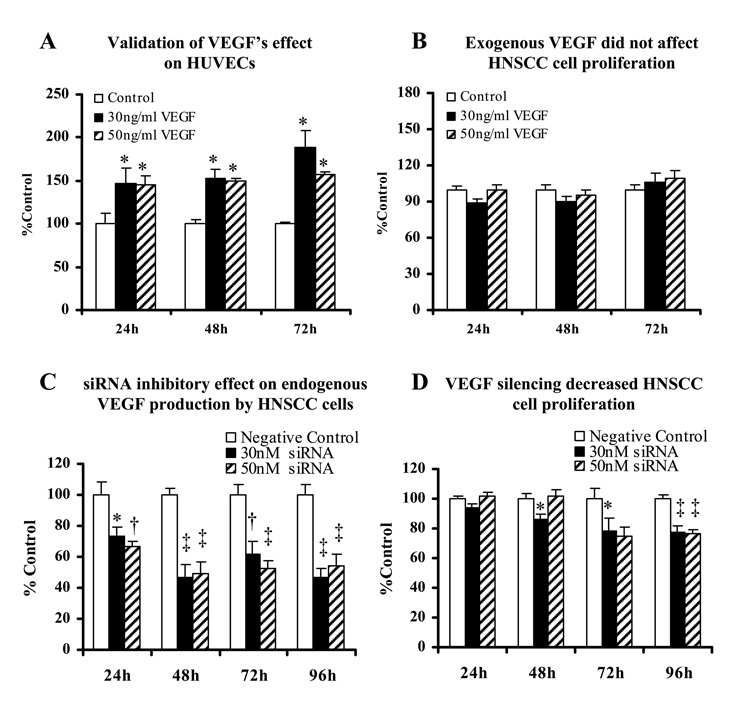
hrVEGF165 increases HUVECs cell proliferation
To confirm the functional activity of hrVEGF165, we first evaluated its mitogenic effects on HUVECs which are established as VEGF target cells [Kanno et al., 2000]. Introduction of hrVEGF165 to HUVECs significantly increased cell proliferation compared to the control HUVECs which received base medium only (Fig. 4A). Based on the preliminary experiments to optimize the effective doses of hrVEGF165 (data not shown), we selected two VEGF doses, 30ng/ml and 50ng/ml, to be used in the subsequent HNSCC cell proliferation studies.
Fig. 4.
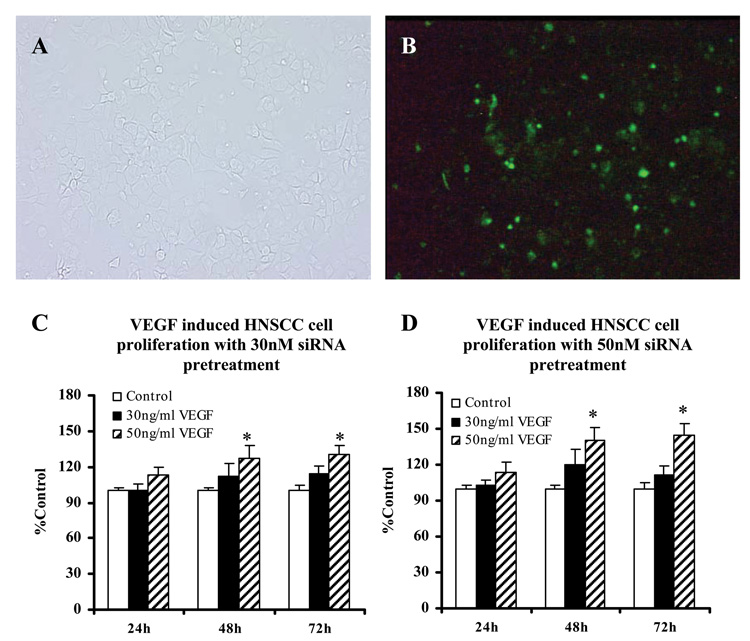
Endogenous VEGF renders HNSCC cells refractory to exogenous VEGF. A: Human umbilical vascular endothelial cells (HUVECs) served as positive control in this study to validate hrVEGF165‘s functional activity. Experimental groups were treated with hrVEGF165 containing medium versus control group received base medium alone. B: Serum deprived non-siRNA-silenced HNSCC cells were challenged with fresh hrVEGF165 every 24 h. Control group received serum free base medium only. C: HNSCC cells were transfected with different concentrations (0, 30nM and 50nM) of anti-VEGF siRNA and incubated at 37°C, 5% CO2. ELISA and MTT assay were performed every 24 h after transfection to determine the amount of VEGF production (presented as pictogram per 104 cells) in the conditioned medium. Values were then normalized to the percentage of negative control for comparisons. D: Impact of VEGF silencing on HNSCC cell proliferation. [Data collected from three HNSCC cell lines, mean ± SE, n=12. *=p<0.05; †=p<0.01; ‡=p<0.001, compared with the control group (Tukey-Kramer multiple comparisons test)]
Endogenous VEGF production renders HNSCC cells refractory to exogenous VEGF
As we previously reported, HNSCC cells produce and secrete abundant endogenous VEGF [Rodrigo et al., 2006]. Accordingly, our results showed introduction of hrVEGF165 to HNSCC cultures did not promote cell proliferation (Fig. 4B). These data suggested that high endogenous VEGF production minimized the effects of exogenous VEGF.
Pre-designed siRNA against VEGF successfully inhibited endogenous VEGF production in HNSCC cells
HNSCC cells were successfully transfected with the FAM-labeled siRNA (>95%) with assistance of a lipid-based transfection agent, NeoFX (Figs. 5A and 5B), and the fluorescence signal of siRNA sustained for more than 96 h. We then transfected the HNSCC cells with pre-designed VEGF siRNA, and determined the amount of VEGF protein (ELISA) per 104 cells (MTT assay), 24, 48, 72, and 96 h after transfection. VEGF production was significantly suppressed by siRNA in all three HNSCC cell lines, and the most prominent inhibition effect was obtained at 48 h (up to 53.5% inhibition) following transfection. Though the silencing effect diminished over time due to cell proliferation, VEGF inhibition remained ≥45.9% at 96 h after transfection (Fig. 4C).
Fig. 5.
Human recombinant VEGF165 promoted HNSCC cell proliferation following suppression of endogenous VEGF production. A and B: HNSCC cells were successfully transfected with NeoFX/FAM-labeled siRNA (72 h after transfection, 200X). C: Serum deprived HNSCC cells were pre-treated with 30nM siRNA to inhibit the endogenous VEGF production 24 h prior to exogenous hrVEGF165 stimulation. D: HNSCC cells were pre-treated with 50nM siRNA. Cell proliferation was determined using the MTT assay. Data of cell numbers from three HNSCC cell lines were normalized to percentage of control group and combined for statistic analysis (mean ± SE, n=20). *=p<0.05. (Unpaired t test)
Blocking VEGF expression decreases HNSCC cell proliferation
Relative to the negative control group which received the empty transfection vector alone, cell proliferation was significantly decreased in VEGF silenced HNSCC cells (Fig. 4D). The greatest suppression of cell growth was observed 96h after siRNA transfection (22.2% and 23.5%, respectively, for the 30nM and 50nM groups).
hrVEGF165 promotes cell proliferation of VEGF-silenced HNSCC cells
A significant dose-dependent increase in siRNA silenced HNSCC cells was observed upon VEGF treatment. Both the amount of siRNA transfected and the amount of VEGF introduced subsequently affected HNSCC proliferation. As can be appreciated in Figs. 5C and 5D, the greatest increase in HNSCC cell growth was observed in cells that received 50nM siRNA + 50mg/ml VEGF. In all groups, hrVEGF165’s pro-proliferative effects on VEGF-silenced HNSCC cells increased over time.
VEGF is comparable or superior to 10% FBS as an HNSCC cellular chemoattractant
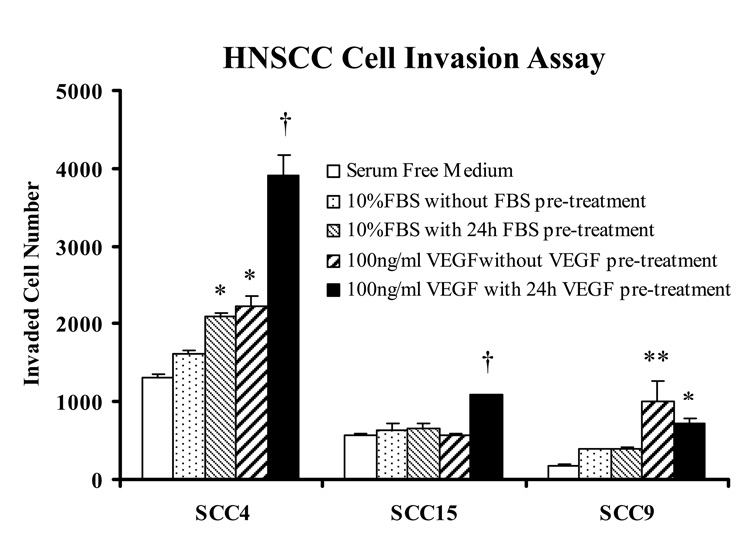
In this component of the study, we explored the role of hrVEGF165 in HNSCC cell migration and invasion through a synthetic basement membrane. Our data demonstrate that 100ng/ml hrVEGF165 exerted equivalent or greater chemoattractive effect relative to 10% FBS in all three HNSCC cell lines (Fig. 6). Also apparent from the data depicted in Figure 6 is the marked heterogeneity among the three tested HNSCC cell lines with regard to invasive capacity.
Fig. 6.
hrVEGF165 is a potent chemoattractant for HNSCC cells. Five experimental groups for each cell line were included in the cell invasion assay: 1), Negative control, 72 h serum deprived HNSCC cells in the insert chamber with SF medium in the lower chamber; 2), Positive control, 72 h serum deprived cells with SF medium+ 10% FBS in the lower chamber serving as chemoattractant; 3), Positive control, 48 h serum deprived and 24 h 10% FBS pretreated cells with 10% FBS chemoattractant; 4), 72 h serum deprived cells with 100ng/ml VEGF chemoattractant; 5), 48 h serum deprived and 24 h 100ng/ml VEGF pretreated cells with 100ng/ml VEGF chemoattractant. Invaded cell numbers were determined after 48 h incubation relative to a cell line matched standard curve (mean ± SE, n=4 for each group. *=p<0.05, **=p<0.01, †=p<0.001, Tukey-Kramer multiple comparisons test).
Prior exposure to hrVEGF165 enhances HNSCC cell invasiveness in two HNSCC cell lines
Two HNSCC cell lines (SCC4 and SCC15) demonstrated significantly increased invasive capacity following a 24h 100ng/ml hrVEGF165 pretreatment with VEGF as the chemoattractant relative to all other groups (negative control, 10% FBS chemoattractant, 10% FBS pretreatment plus 10% FBS chemoattractant, and 100ng/ml VEGF chemoattractant alone) (Fig. 6). Cell invasion was increased by 118.8% and 89.6% respectively in SCC4 and SCC15 cells, compared with the group that received VEGF only as a chemoattractant (Fig. 6).
hrVEGF165 modestly increases gelatinases release by HNSCC cells
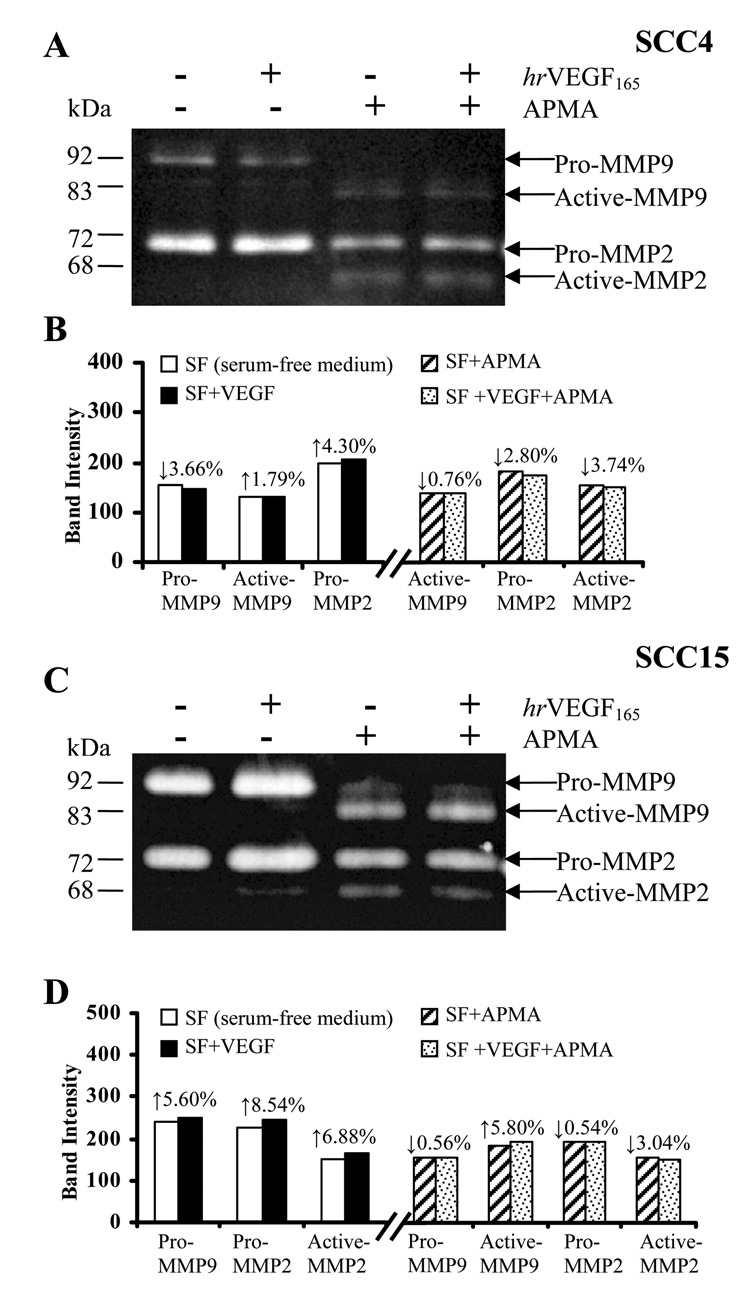
To explore potential mechanisms whereby VEGF enhances HNSCC invasiveness, we evaluated the release of the basement membrane degrading gelatinases MMP2 and MMP9 (Matrix Metalloproteinases) in conditioned media following VEGF stimulation. Gelatin zymography results showed a cell line associated release pattern of both latent and active MMP2 and MMP9 (Fig. 7A and 7C). As would be anticipated, introduction of APMA resulted in significant conversion of pro-MMPs to their respective activated isoforms (Figs. 7A and 7C). Release of the latent pro-MMP2 was increased by 4.30% and 8.54% in SCC4 and SCC15 respectively after 24h VEGF treatment. VEGF’s modulation of pro-MMP9 release was cell line dependent, as VEGF increased SCC15 pro-MMP9 by 5.60% while decreased its release by 3.66% in SCC4 (Figs. 7B and 7D).
Fig. 7.
Treatment of HNSCC cells with hrVEGF165 modestly increased gelatinase release. A and C: HNSCC cells (SCC4 and SCC15) were serum deprived for 48 h followed by fresh treatment of serum-free medium with or without presence of 100ng/ml VEGF for 24 h. In selected groups, the conditioned media were pre-incubated with the pro-MMP activator, APMA, for 2 h. Gelatin zymography were then conducted to demonstrate released gelatinases (MMP2 and MMP9) levels in the conditioned media. B and D: Densitometry analyses were performed using Kodak 1D3 image analysis software.
DISCUSSION
The assumption of mesenchymal phenotypic properties via the EMT by carcinomas enhances cell invasion and facilitates cancer progression [Thiery et al., 1999; Christiansen et al., 2006; Gotzmann et al., 2004; Chung et al., 2006]. Our lab previously reported that the angiostatic agent, endostatin, which was presumed to target endothelial cells, also significantly inhibited HNSCC cell migration and invasion [Wilson et al., 2003]. Similarly, in this current study, we demonstrated that HNSCC cells exhibit an “endothelioid” phenotype, as these cells serve as both effectors and targets of VEGF. Our data, in conjunction with clinical evidence which shows retention of VEGF receptors in HNSCC tumors [Kyzas et al., 2005; Lalla et al., 2003], imply that a plasticity in the HNSCC phenotype underlies, at least in part, its aberrant progression, invasion and metastasis.
Due to its multifaceted biologic functions, VEGF is regarded as an important tumor promoter [Folkman, 1992]. Doses of VEGF used in this study, which ranged from 30ng/ml to 100ng/ml (approximately 1.5nM to 5nM), were selected based on the results of our preliminary experiments. hrVEGF165 (293-VE/CF, R&D, same as used in this current study) has been shown to have a wide bioactivity range [0.05nM to 250nM (1ng/ml to 5000ng/ml)] with a maximum effect at 25nM (500ng/ml) on HUVECs, as reported by Pan et al. [2007].
The delineation of KDR and Flt-1’s distinctive roles in VEGF signaling in cancer cells remains elusive. Studies employing breast cancer cells revealed that both KDR and Flt-1 may have the potential to activate downstream kinases in the VEGF intracellular signaling cascade [Lee et al., 2007; Wu et al., 2006; Huh et al., 2005; Weigand et al., 2005]. Our data demonstrated that SCC4 cells, which have highest level of Flt-1 and greatest levels of KDR activation, are also most responsive to VEGF in the cell invasion assay. These data strongly imply a potential co-contributory role for Flt-1 in conjunction with KDR towards augmentation of the HNSCC cellular aggressive, invasive phenotype. Studies are ongoing to further characterize this VEGF-associated signaling mechanism in HNSCC cells. Furthermore, we speculate that the high “baseline” phosphorylated Erk1/2 in non-siRNA silenced SCC15 cells reflects endogenous VEGF production rendering these cells relatively refractory to signaling induction by exogenous VEGF.
The contribution of ROS as intracellular signaling mediators is an area of intense investigation [Finkel, 1998; Rhee et al., 2000]. Introduction of VEGF induces intracellular ROS generation in endothelial cells [Colavitti et al., 2002]. Reciprocally, intracellular levels of ROS may also regulate VEGF expression [Xia et al., 2007]. Our data, which show VEGF initiated comparable ROS generation in HNSCC cells as H2O2, imply that ROS function in the VEGF receptor(s)-mediated signaling pathway(s) in HNSCC cells.
Our results showed that HNSCC cell proliferation was significantly decreased by VEGF siRNA silencing; implying the presence of an autocrine-paracrine VEGF growth loop. Similarly, down-regulation of VEGF expression also induced apoptosis and decreased cell proliferation in some breast cancer cell lines [Lee et al., 2007; Weigand et al., 2005]. Furthermore, once endogenous HNSCC VEGF production was reduced by siRNA, a dose dependent, exogenous VEGF-mediated increase in cell proliferation occurred. While statistically significant, the actual percentage changes in cell proliferation induced by VEGF were relatively modest, which likely reflects the contribution of multiple growth factors in HNSCC cell proliferation. These findings suggest that in addition to its angiogenic function, VEGF fulfills other roles by maintaining proliferation, enhancing survival, and increasing invasion of carcinomas.
VEGF was found to have similar or greater chemoattractant efficacy as 10% FBS in all HNSCC cell lines evaluated. Pretreatment with VEGF increased the abilities of 2 of 3 HNSCC cell lines to invade a synthetic basement membrane. Gelatin zymograghy, however, did not demonstrate the anticipated comparable increase of the basement membrane degrading gelatinases MMP-2 or MMP-9. These data imply that VEGF pretreatment may concurrently up-regulate additional enzymes such as the membrane-bound metalloproteinases (MT-MMPs), which are critical for invasion of basement membranes and have been revealed to be expressed in HNSCC cells and tumors [Bassi et al., 2003]. The complexity of the parameters that affect HNSCC invasion were demonstrated by previous investigations from our lab [Pei et al., 2006; Wilson et al., 2003]. While N-acctylcysteine-mediated inhibition of gelatinase activation and function suppressed HNSCC invasion [Pei et al., 2006], introduction of a tropomysin binding agent (endostatin) [Wilson et al., 2003] was necessary to maximally inhibit HNSCC invasive capacity. Based on our previous and current findings, we speculate that VEGF’s pro-invasive effects reflect upregulation of multiple, complementary functions including augmentation of basement membrane invasion and enhancement of cell motility, necessary for cell invasion. Consequently, studies to assess VEGF’s effects on HNSCC gene expression profiles are ongoing.
Interestingly, unlike the cell proliferation assay, non-siRNA treated HNSCC cells exhibited significantly increased intracellular signaling and invasiveness in response to exogenous VEGF. We propose two possible reasons for these findings. Firstly, due to the short duration (<30min) of the signaling assays, there is insufficient time for accumulation of endogenous VEGF to render these cells refractory to exogenous VEGF. Secondly, as VEGF’s chemoattractive and mitogenic roles are distinct; the corresponding signaling pathways are likely different.
In conclusion, our results demonstrate that in addition to its paracrine function essential for tumor-associated angiogenesis, VEGF also plays an important autocrine function by directly enhancing HNSCC cell mitogenesis and invasiveness. These data introduce the prospect that agents which interfere with HNSCC VEGF production and responsiveness could have a biphasic therapeutic effect by concurrently reducing angiogenesis and HNSCC tumorigenesis.
ACKNOWLEDGEMENTS
This study is supported by NCI R01CA95901, NCI R21CA111210 (Dr. Susan R. Mallery).
Grant Information:
Contract grant sponsor: NIH; Contract grant number: NCI R01CA95901 and NCI R21 CA111210.
REFERENCES
- Artese L, Rubini C, Ferrero G, Fioroni M, Santinelli A, Piattelli A. Microvessel density (MVD) and vascular endothelial growth factor expression (VEGF) in human oral squamous cell carcinoma. Anticancer Res. 2001;21:689–695. [PubMed] [Google Scholar]
- Bassi DE, Mahloogi H, Lopez De Cicco R, Klein-Szanto A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am J Pathol. 2003;162:439–447. doi: 10.1016/s0002-9440(10)63838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates RC, Goldsmith JD, Bachelder RE, Brown C, Shibuya M, Oettgen P, Mercurio AM. Flt-1-dependent survival characterizes the epithelial-mesenchymal transition of colonic organoids. Curr Biol. 2003;13:1721–1727. doi: 10.1016/j.cub.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Chin D, Boyle GM, Porceddu S, Theile DR, Parsons PG, Coman WB. Head and neck cancer: Past, present and future. Expert Rev Anticancer Ther. 2006;6:1111–1118. doi: 10.1586/14737140.6.7.1111. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, Ang KK, El-Naggar AK, Zanation AM, Cmelak AJ, Levy S, Slebos RJ, Yarbrough WG. Cancer Res. 2006;66:8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 suppl 3:11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2007;18:47–51. [PubMed] [Google Scholar]
- Fury MG, Zahalsky A, Wong R, Venkatraman E, Lis E, Hann L, Aliff T, Gerald W, Fleisher M, Pfister DG. A phase II study of SU5416 in patients with advanced or recurrent head and neck cancers. Invest New Drugs. 2007;25:165–172. doi: 10.1007/s10637-006-9011-x. [DOI] [PubMed] [Google Scholar]
- Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W. Molecular aspects of epithelial cell plasticity: Implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- Gourin CG, McAfee WJ, Neyman KM, Howington JW, Podolsky RH, Terris DJ. Effect of comorbidity on quality of life and treatment selection in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115:1371–1375. doi: 10.1097/01.mlg.0000167983.32017.64. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hasina R, Lingen MW. Angiogenesis in oral cancer. J Dent Educ. 2001;65:1282–1290. [PubMed] [Google Scholar]
- Huh JI, Calvo A, Stafford J, Cheung M, Kumar R, Philp D, Kleinman HK, Green JE. Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms. Oncogene. 2005;24:790–800. doi: 10.1038/sj.onc.1208221. [DOI] [PubMed] [Google Scholar]
- Johnstone S, Logan RM. The role of vascular endothelial growth factor (VEGF) in oral dysplasia and oral squamous cell carcinoma. Oral Oncol. 2006;42:337–342. doi: 10.1016/j.oraloncology.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Potential autocrine function of vascular endothelial growth factor in head and neck cancer via vascular endothelial growth factor receptor-2. Mod Pathol. 2005;18:485–494. doi: 10.1038/modpathol.3800295. [DOI] [PubMed] [Google Scholar]
- Lalla RV, Boisoneau DS, Spiro JD, Kreutzer DL. Expression of vascular endothelial growth factor receptors on tumor cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:882–888. doi: 10.1001/archotol.129.8.882. [DOI] [PubMed] [Google Scholar]
- Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Kumagai S, Kawashiri S, Kojima K, Kakihara K, Yamamoto E. Immunohistochemical study of tumour angiogenesis in oral squamous cell carcinoma. Oral Oncol. 1997;33:369–374. doi: 10.1016/s1368-8375(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: Animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- Neuchrist C, Erovic BM, Handisurya A, Steiner GE, Rockwell P, Gedlicka C, Burian M. Vascular endothelial growth factor receptor 2 (VEGFR2) expression in squamous cell carcinomas of the head and neck. Laryngoscope. 2001;111:1834–1841. doi: 10.1097/00005537-200110000-00031. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- Pei P, Horan MP, Hille R, Hemann CF, Schwendeman SP, Mallery SR. Reduced nonprotein thiols inhibit activation and function of MMP-9: Implications for chemoprevention. Free Radic Biol Med. 2006;41:1315–1324. doi: 10.1016/j.freeradbiomed.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000:PE1. 2000 doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2007;21:44–52. doi: 10.1038/sj.leu.2404402. [DOI] [PubMed] [Google Scholar]
- Rodrigo KA, Rawal Y, Renner RJ, Schwartz SJ, Tian Q, Larsen PE, Mallery SR. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr Cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano D, Kawakami M, Fujita K, Kimura M, Yamashita Y, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Antitumor effects of ZD6474 on head and neck squamous cell carcinoma. Oncol Rep. 2007;17:289–295. [PubMed] [Google Scholar]
- St John MA, Abemayor E, Wong DT. Recent new approaches to the treatment of head and neck cancer. Anticancer Drugs. 2006;17:365–375. doi: 10.1097/01.cad.0000198913.75571.13. [DOI] [PubMed] [Google Scholar]
- Stewart M, Turley H, Cook N, Pezzella F, Pillai G, Ogilvie D, Cartlidge S, Paterson D, Copley C, Kendrew J, Barnes C, Harris AL, Gatter KC. The angiogenic receptor KDR is widely distributed in human tissues and tumours and relocates intracellularly on phosphorylation an immunohistochemical study. Histopathology. 2003;43:33–39. doi: 10.1046/j.1365-2559.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- Weigand M, Hantel P, Kreienberg R, Waltenberger J. Autocrine vascular endothelial growth factor signalling in breast cancer: evidence from cell lines and primary breast cancer cultures in vitro. Angiogenesis. 2005;8:197–204. doi: 10.1007/s10456-005-9010-0. [DOI] [PubMed] [Google Scholar]
- Wilson RF, Morse MA, Pei P, Renner RJ, Schuller DE, Robertson FM, Mallery SR. Endostatin inhibits migration and invasion of head and neck squamous cell carcinoma cells. Anticancer Res. 2003;23:1289–1295. [PubMed] [Google Scholar]
- Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, Hicklin DJ. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- Yang AD, Camp ER, Fan F, Shen L, Gray MJ, Liu W, Somcio R, Bauer TW, Wu Y, Hicklin DJ, Ellis LM. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bhola NE, Lui VW, Siwak DR, Thomas SM, Gubish CT, Siegfried JM, Mills GB, Shin D, Grandis JR. Antitumor mechanisms of combined gastrin-releasing peptide receptor and epidermal growth factor receptor targeting in head and neck cancer. Mol Cancer Ther. 2007;6:1414–1424. doi: 10.1158/1535-7163.MCT-06-0678. [DOI] [PubMed] [Google Scholar]