Fig. 5. Specificity of interaction between Rpn10 and the Ubl domain containing protein, Dsk2.
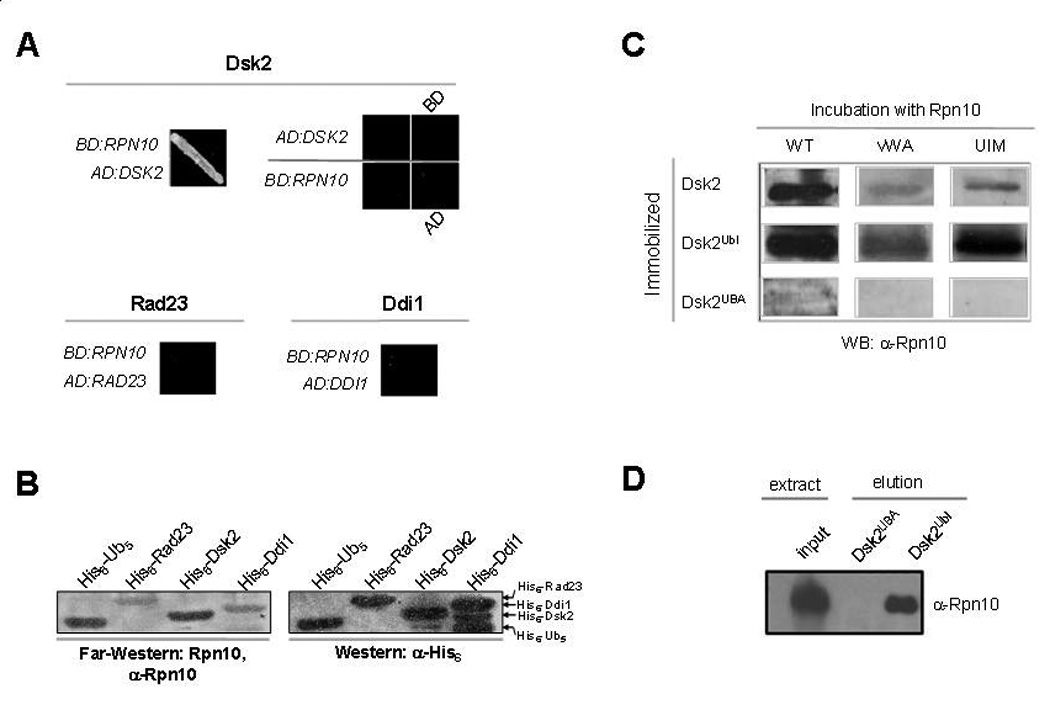
A. Dsk2 and Rpn10 interact by yeast two-hybrid. Association of Rpn10 with the Ubl-containing proteins, Dsk2, Rad23, or Ddi1 was tested in vivo by transforming all possible combinations of bait versus prey constructs and assaying for growth on –His–Trp dropout selective media supplemented with 7.5mM 3-aminotriazol. Representative results are shown. A lone combination, BD:RPN10 with AD:DSK2, supported growth.
B. Direct protein-protein interaction of Rpn10 with Dsk2. 30 pmol of Ub/Ubl-containing proteins were immobilized on a nitrocellulose membrane. Migration pattern and amounts of immobilized proteins was evaluated by immunoblotting with anti-His6 antiserum (right panel). In parallel, a similar membrane was incubated with 2µg/ml of recombinant Rpn10, washed, and immunoblotted against residually bound Rpn10 (left panel). Association of Rpn10 with polyUb is shown as a positive control.
C. The Ubl domain of Dsk2 interacts with the UIM of Rpn10. Dsk2 or its structural domains were subjected to Far-Western analysis using either full length Rpn10 or its truncated versions as in panel B.
D. The Ubl domain of Dsk2 is sufficient to precipitate Rpn10 from whole cell extract. Whole cell extract was applied to an affinity column generated from crosslinked Dsk2Ubl or Dsk2UBA coupled to activated Sepharose resin. After stringent washes, bound proteins were eluted with 8M urea and assayed from presence of Rpn10 by immunoblotting.
