Figure 3.
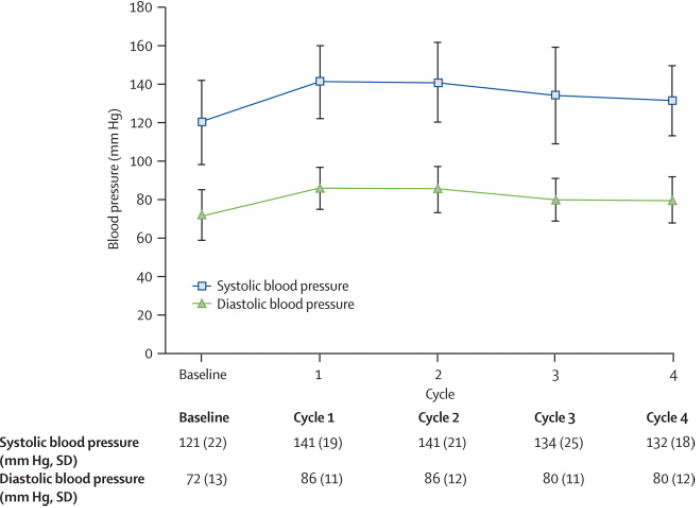
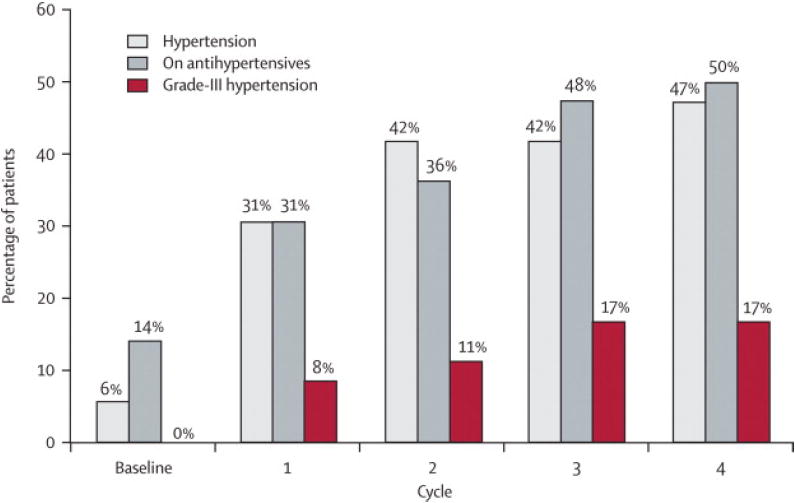
Effect of sunitinib 50 mg; 4 wks on/2 wks off on blood pressure.
A. Changes in mean systolic (SBP) and diastolic blood pressure (DBP) during the first 4 cycles of therapy. By the first cycle, SBP and DBP had significantly increased from baseline, and remained elevated through cycle 4.
B. Cumulative percentage of patients diagnosed with hypertension and on anti-hypertensive medication during the first four cycles (24 weeks) of sunitinib treatment (n=36). Hypertension was defined as > 150 mmHg systolic and/or > 100 mmHg diastolic. Grade III hypertension denotes patients who required more than one anti-hypertensive medication and/or required an increase in anti-hypertensive medication (National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0), prescribed at the discretion of the caring physician. By Cycle 4, almost half the cohort developed HTN, and 50% of patients were on anti-hypertensive medication.
