Fig. 7.
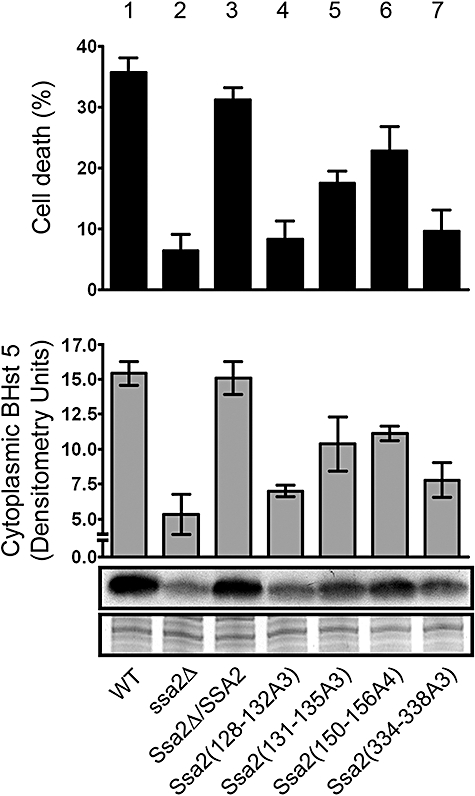
Mutations in Ssa2p Hst 5-binding epitopes reduce Hst 5 uptake and cytotoxicity. C. albicans constructs of SSA2 mutations were tested for sensitivity to Hst 5 (top) and ability to translocate Hst 5 to the cytosol (bottom). Candidacidal assays were performed by incubating cells with 15 μM Hst 5 for 1 h at 30°C, and percentage cell death was calculated compared with untreated cells. Cytosolic translocation of Biotin-Hst 5 (15 μM) was measured for each C. albicans construct using the same conditions as for candidacidal assays. Cytosolic proteins (10 μg) from each construct were subjected to 16% Tricine SDS-PAGE, immunoblotted with Streptavidin-HRP to detect BHst 5, and quantified. Control proteins from each construct are shown to verify equal protein loading. Mutations in Ssa2(128−132A3) resulted in complete loss of killing and translocation (lane 4) equivalent to the ssa2Δ knockout, while mutations in Ssa2(334−338A3) (lane 7) had significant loss of cytotoxic and transport functions. Mutations in Ssa2(131−135A3) and Ssa2(150−156A4) (lane 5 and lane 6) resulted in mild to moderate loss of function.
