Abstract
Lung fibrosis is a recognized feature of many chronic lung diseases and is central to the pathogenesis of idiopathic pulmonary fibrosis, a disease that carries a prognosis worse than many cancers. Current research into this condition is defining the key pathways of activation either in resident fibroblasts, matrix-producing cells derived from circulating fibrocytes, or epithelial cells that appear to transdifferentiate to fibroblast-like cells. The downstream signaling pathways are also being delineated as well as the gene interactions leading to altered cell phenotype. These studies have led to an appreciation that multiple pathways, including inflammatory and coagulation cascades, are involved in the pathogenesis of idiopathic pulmonary fibrosis. As these facts come to light, we are exploring promising new approaches to treat fibroses and halt the inexorable progression that is a feature of these disorders. This article reviews these findings and our current concepts of the key molecular events leading to tissue damage and excessive matrix deposition in lung fibrosis. It also highlights the need for new studies to delineate alternative pathogenetic mechanisms and integrate these pathways so we have a framework to better understand their importance in individual patients.
Keywords: fibroblasts, lung fibrosis, matrix
Chronic lung diseases are a major cause of morbidity and mortality and an enormous burden on world health systems. We now recognize that there are often common mechanisms in these chronic diseases and that new treatments developed in one setting may have application to others. A good example of this is the fibrosis that is central to idiopathic pulmonary fibrosis (IPF) but is now recognized to be an important feature of asthma and chronic obstructive pulmonary disease (1, 2). IPF has a poor prognosis (median survival, 3–5 yr) and an increasing incidence, and current therapies are ineffective (3, 4). A central feature of this disease is the destruction and remodeling of the lung's supportive matrix, leading to severely compromised gas exchange. This article reviews our current concepts of the pathogenesis of IPF and in so doing highlights the emerging approaches to treat IPF and other chronic lung diseases in which fibrosis is a component.
FIBROBLASTS AND THE FIBROTIC PHENOTYPE
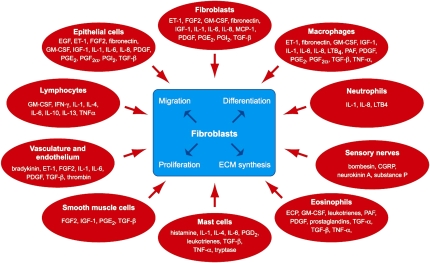
It is now well recognized that the lung is actively synthesizing and degrading a diverse group of matrix components and the rates of these processes are rapid in normal tissues (5, 6). Many cell types are involved, but predominantly mesenchymal cells (fibroblasts, myofibroblasts, and smooth muscle cells) are responsible for this turnover (reviewed in References 1 and 7). Fibroblasts are widely distributed in all lung structures and play a key role in matrix homeostasis. For example, in response to growth factors or mechanical stimuli, these cells are capable of generating more than 5,000 molecules of procollagen per cell per minute (8, 9). They are also in communication with a large number of different cell types and respond to a host of cytokines and growth factors (Figure 1). More recent studies using microarray technologies have further reiterated the diversity of this response. We have recently profiled human fetal lung fibroblast global gene expression in response to transforming growth factor (TGF)-β1 using oligonucleotide microarrays and reported that at least 150 genes are up-regulated across several major functional categories, including genes involved in cytoskeletal reorganization, matrix formation, metabolism and protein biosynthesis, cell signaling, proliferation, survival, and gene transcription (10). This included 80 that were not previously known to be TGF-β1 responsive. Such a profound and diverse transcriptional response is also reflected in vivo, with studies of pulmonary fibrosis in human and animal models reporting that almost 500 genes are differentially expressed more than twofold, including a large cluster of diverse matrix and matrix-related genes (11, 12).
Figure 1.
Fibroblasts and factors. CGRP = calcitonin gene-related peptide; ECP = eosinophil chemotactic protein; EGF = epidermal growth factor; ET-1 = endothelin-1; FGF2 = fibroblast growth factor 2; GM-CSF = granulocyte-macrophage colony–stimulating factor; IGF-1 = Insulin-like growth factor-1; LTB4 = leukotriene B4; MCP-1 = monocyte chemotactic protein-1; PAF = platelet activating factor; PDGF = platelet-derived growth factor; PGD2 = prostaglandin D2; PGE2 = prostaglandin E2; PGF2α = prostaglandin F2α; PGI2 = prostacyclin; TGF-α = transforming growth factor-α; TGF-β = transforming growth factor-β; TNF-α = tumor necrosis factor-α. Reprinted by permission from Reference 1.
PROFIBROGENIC CYTOKINES AND PULMONARY FIBROSIS
A large number of mediators produced by many different cell types are known to promote fibroblast proliferation, collagen synthesis, migration, and differentiation (for review, see References 1, 13, and 14, and Figure 1). TGF-β is the most potent profibrotic mediator characterized to date and is a central player in several remodeling diseases, including asthma (15) and pulmonary fibrosis (16). Blocking the action of TGF-β by a number of strategies has been shown to ameliorate experimental pulmonary fibrosis. On this basis, pharmaceutical companies are developing and testing several classes of TGF-β blockers, including inhibitors of latent TGF-β activation, TGF-β blocking antibodies, and receptor kinase inhibitors, in the hope of better treating human fibrosis. A serious caveat, however, is the role TGF-β plays as an inhibitor of immune responses or as a tumor suppressor (17). For this reason, there are concerns that blocking TGF-β may lead to undesired side effects but thus far these reservations appear to be unfounded (reviewed in Reference 18).
Many other cytokines in addition to TGF-β are believed to play roles in the pathogenesis of IPF (14). Based on a modified Koch's postulate, we proposed some time ago a criterion to evaluate the likelihood of any one individual cytokine/mediator playing a role in IPF (19). These criteria include the presence in the lung of patients with IPF, profibrotic properties in vitro, and amelioration when the action of the cytokine is blocked in animal models. Many cytokines/mediators, including tumor necrosis factor (TNF)-α, endothelin (ET)-1, platelet-derived growth factor (PDGF), insulin-like growth factor (IGF)-1, IL-1, and IL-13 fulfill these criteria and inhibitors are currently being tested in humans.
ENDOTHELIN AND ANGIOTENSIN: VASOCONSTRICTORS THAT REGULATE REMODELING AND FIBROSIS
It has been observed that vasoconstrictors often induce remodeling and fibrosis, whereas vasodilators are inhibitors. For example, the vasoconstrictors ET-1 and angiotensin II both exhibit profibrotic features in vitro (20, 21), and receptor antagonists of these agents have shown some success in blocking fibrosis in animal models. Furthermore, a common polymorphism in angiotensin converting enzyme has been shown to influence outcome in patients with acute respiratory distress syndrome (22), a disease in which fibrosis is often a component (23). The relevance of these studies to humans remains uncertain and will await the results of ongoing trials with drugs blocking these pathways.
CYTOKINES AND LIPID MEDIATORS AS INHIBITORS OF FIBROBLAST FUNCTION AND FIBROSIS
Fibrosis occurs if the normal homeostatic balance is perturbed, resulting in an excessive production of profibrotic mediators that then activate fibroblasts (14). This concept has led us to consider ways of changing this balance in patients with fibrotic disorders. The focus has mostly been on inhibition of profibrotic cytokines, but there is also considerable interest in strategies to up-regulate antifibrotic molecules. This was given impetus by the reports in a small group of patients that IFN-γ was an effective treatment for IPF (24), although this early promise was not borne out in a recent large multicenter trial (25). Another molecule of interest is prostaglandin E2 (PGE2), a product of cyclooxygenase (COX) catalyzed arachidonic acid metabolism, which is an inhibitor of fibroblast proliferation and collagen deposition (26, 27). PGE2 production is reduced in fibroblasts from patients with lung fibrosis after stimulation with mediators such as IL-1 (28) or TGF-β (29), and this is due to a decreased capacity to up-regulate COX2. Furthermore, COX2-deficient mice are more susceptible to bleomycin-induced pulmonary fibrosis (30), supporting the hypothesis that there is a defect in the COX2–PGE2 axis in fibrosis. We have also recently shown that a functional promoter polymorphism in the COX2 gene (PTGS2), which reduces gene expression (31), is associated with susceptibility to sarcoidosis (32). Moreover, the association is most common in those patients with persistent progressive disease who are most likely to develop pulmonary fibrosis (32). These data highlight the importance of gene interactions and also suggest that therapeutic strategies to overexpress antifibrotic molecules might be fruitful. One possibility may be a gene delivery approach, although there are clearly concerns about safety of this approach when viral vectors are used. To circumvent these concerns, we have explored an integrin-targeting gene delivery system, composed of a cationic liposome, an integrin binding peptide, and plasmid DNA, which shows high delivery efficiency while avoiding the immune and inflammatory effects associated with the use of adenoviral vectors (33).
PROTEASES IN THE REGULATION OF FIBROBLAST FUNCTION AND REMODELING
The serine and matrix metalloproteinases have long been assumed to play key roles in emphysema in which degradation of matrix and destruction of parenchymal lung structures are a feature. However, there is increasing evidence that these molecules are also important in the pathogenesis of acute lung injury and pulmonary fibrosis (34). In this context, inhibitors of neutrophil elastase have been shown to inhibit lung injury and fibrosis (35–37), and we have recently demonstrated that mice deficient for this proteinase are protected from lung fibrosis (38).
Proteinases of the coagulation cascade, including factor VIIa, factor Xa, and thrombin, exert proinflammatory and profibrotic effects, and likely play key roles in acute lung injury and remodeling disorders of the lung (39–42). These proteinases exert their cellular effects via interaction with four proteinase-activated receptors, PAR1–PAR4, with the coagulation proteinases of the extrinsic pathway targeting all four of these receptors. In terms of influencing fibroblast function, we and others have shown that PAR1, the high-affinity thrombin receptor, is the major receptor by which thrombin (39, 40) and factor Xa (43) exert their potent profibrotic effects. Thus, PAR1 has emerged as a promising new target to prevent fibrosis both in the setting of IPF and acute respiratory distress syndrome. Furthermore, thrombin inhibition partially blocks experimental fibrosis (44), and mice deficient for PAR1 are protected from lung inflammation, pulmonary edema, and lung collagen accumulation after bleomycin injury (45, 46).
MECHANICAL FORCES AND FIBROBLAST FUNCTION
The potent actions of mechanical forces on influencing fibroblast phenotype have long been recognized in studies of muscle (47), skin (48), and the cardiovasculature (49). More recently, the potential role for mechanical forces in altering lung cell phenotype has also been highlighted (50), although we still know little of the mechanisms of mechanosensing and the subsequent molecular pathways involved. In the context of fibrosis, it is of interest that mechanical forces promote matrix production and that this effect is mediated via multiple cell surface receptors and intracellular signaling pathways (51–53) and involves autocrine actions of TGF-β (9). The relevance of these pathways to lung fibrosis remains to be elucidated.
APOPTOSIS AND PULMONARY FIBROSIS
Apoptotic pathways are key to the resolution of inflammation and fibrosis after lung injury (54). In pulmonary fibrosis, there is some evidence that fibroblasts are resistant to apoptosis (55) and that epithelial cells may be more susceptible to this process (56). Thannickal and Horowitz (57) have reviewed the evidence for these changes and suggested that this “apoptosis paradox” is central to the pathogenesis of IPF. In experimental models of IPF, the data suggest that inhibitors of the proapoptotic molecules, Fas or the caspases, inhibit fibrosis (58, 59), consistent with epithelial cell apoptosis being an important event. The mechanisms driving these events are uncertain, although TGF-β is again emerging as a central player (60).
CELL PLASTICITY: EPITHELIAL–MESENCHYMAL TRANSITION AND BLOOD FIBROCYTES
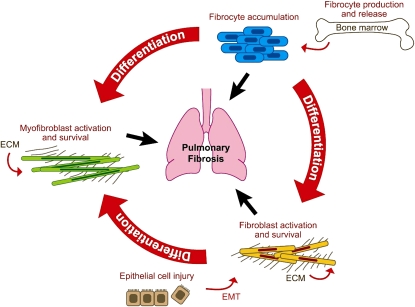
The pathways leading to fibrosis are now recognized to involve considerable cell plasticity, with the excessive numbers of profibrotic cells potentially being derived from several sources (Figure 2). Epithelial–fibroblast interactions may be central to remodeling both in the airways (61) and in the fibrotic foci within the lung parenchyma of patients with IPF (62, 63). Epithelial cells can transdifferentiate into mesenchymal cells with a profibrotic phenotype (64, 65). They can also release many profibrotic cytokines, including TGF-β, IGF-1, and ET-1, which stimulate fibroblast proliferation and procollagen production. Furthermore, these cells may play key roles in the activation of growth factors via cell surface integrins. Considerable interest has focused on the epithelial cell integrin αvβ6. Mice deficient in the β6 subunit are protected from pulmonary fibrosis and lack the ability to activate TGF-β (66). Furthermore, recent studies suggest that PAR1 activation leads to the generation of active TGF-β via an αvβ6-dependent mechanism (46). These data, taken together with the paucity of inflammatory cells in late-stage IPF and the ineffectiveness of current antiinflammatory drugs, have led to the suggestion that remodeling and fibrosis may proceed independently of inflammation and that epithelial cells have a central role.
Figure 2.
Sources of fibroblasts and myofibroblasts in pulmonary fibrosis. Blood-borne fibrocytes, derived from bone marrow stem cells, target to the lung where they may play roles in elaborating matrix. Fibroblasts may also be derived from the transdifferentiation of epithelial cells, a process termed epithelial–mesenchymal transition (EMT). Finally, resident fibroblasts are also capable of transdifferentiation into myofibroblasts, which exhibit both a contractile and matrix-producing phenotype. The relative importance of these pathways in human disease is yet to be elucidated. ECM = extracellular matrix. Adapted by permission from Reference 71.
Recent studies have also suggested that fibrocytes, bone marrow–derived blood cells expressing type I collagen, are targeted to the lung both in experimental disease (67) and in human pulmonary fibrosis (68). Furthermore, several different chemokines have been implicated in the recruitment process (69). A better understanding of this process and the molecular interactions that underpin it are urgently needed.
CONCLUSIONS
This article outlines our current concepts for the pathogenesis of IPF, a disease characterized by excessive matrix deposition at fibrotic foci within the lung parenchyma. Studies from a large number of groups have led to an appreciation that there are multiple pathways involved (recently reviewed in Reference 70). Furthermore, in some cases, the molecular events are now well described, leading to new drugs that are being tested in clinical trials. Many challenges remain; at this point in time, we have no adequate drugs to treat IPF and undoubtedly new pathways remain to be discovered. It will also be vital in the future to develop ways to assess the importance of these pathways in individual patients and at different stages of the disease process. Studies of genetic and epigenetic mechanisms that increase susceptibility to fibrosis are still in the very earliest stages and more effort in this area is urgently needed. As these new studies are performed and new facts come in, we remain hopeful that new avenues will emerge to treat lung diseases in which fibrosis is an important component.
The Centre for Respiratory Research, University College London receives major support from the Wellcome Trust and the Medical Research Council of Great Britain.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.McAnulty RJ, Laurent GJ. Fibroblasts. In: Barnes P, Drazen J, Rennard S, Thomson N, editors. Asthma and COPD: basic mechanisms and clinical management. London: Academic Press; 2002. pp. 139–144.
- 2.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134–155. [DOI] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277–284. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Toews GB, White ES, Lynch JP, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 5.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol 1987;252:C1–C9. [DOI] [PubMed] [Google Scholar]
- 6.Dunsmore SE, Chambers RC, Laurent GJ. Matrix proteins. In: Gibson GJ, Geddes DM, Costabel U, Sterk PJ, Corrin B, editors. Respiratory medicine, 3rd ed. Oxford (UK): Saunders; 2003. pp. 82–92.
- 7.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 2007;39:666–671. [DOI] [PubMed] [Google Scholar]
- 8.McAnulty RJ, Campa JS, Cambrey AD, Laurent GJ. The effect of transforming growth factor β on rates of procollagen synthesis and degradation in vitro. Biochim Biophys Acta 1991;1091:231–235. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl GE, Chambers RC, Papakrivopoulou J, Dawson SJ, Jacobsen MC, Bishop JE, Laurent GJ. Activation of fibroblast procollagen α 1(I) transcription by mechanical strain is transforming growth factor-β-dependent and involves increased binding of CCAAT-binding factor (CBF/NF-Y) at the proximal promoter. J Biol Chem 2002;277:6153–6161. [DOI] [PubMed] [Google Scholar]
- 10.Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-β1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol 2003;162:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA 2000;97:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coker RK, Laurent GJ. Anti-cytokine approaches in pulmonary fibrosis: bringing factors into focus. Thorax 1997;52:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J 1998;11:1218–1221. [DOI] [PubMed] [Google Scholar]
- 15.Howell JE, McAnulty RJ. TGF-β: its role in asthma and therapeutic potential. Curr Drug Targets 2006;7:547–565. [DOI] [PubMed] [Google Scholar]
- 16.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-β1, -β2, and -β3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997;150:981–991. [PMC free article] [PubMed] [Google Scholar]
- 17.Shull MM, Ormsby I, Kier AB, Pawlowsky S, Diebold RJ, Yin M, Allen R, Sidman C, Preotzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 1992;359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAnulty RJ. In: Polosa R, Holgate ST, editors. Therapeutic strategies asthma, modern therapeutic targets. Oxford, UK: Clinical Publishing; 2007. pp. 15–32.
- 19.Coker RK, Laurent GJ. Pathogenesis of pulmonary fibrosis: implications for pharmacological intervention. In: Walters EH, du Bois RM, editors. Immunology and interstitial lung disease. London: Chapman & Hall; 1995. pp. 19–36.
- 20.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med 2000;161:1999–2004. [DOI] [PubMed] [Google Scholar]
- 21.Peacock A, Dawes KE, Shock A, Gray AJ, Reeves JT, Laurent GJ. Endothelin-1 and endothelin-3 induce chemotaxis and replication of pulmonary artery fibroblasts. Am J Respir Cell Mol Biol 1992;7:492–499. [DOI] [PubMed] [Google Scholar]
- 22.Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ, Humphries SE, Hill MR, Laurent GJ. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med 2002;166:646–650. [DOI] [PubMed] [Google Scholar]
- 23.Marshall R, Bellingan GJ, Laurent GJ. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax 1998;53:815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999;341:1264–1269. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon gamma-1β in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 26.McAnulty RJ, Chambers RC, Laurent GJ. Regulation of fibroblast procollagen production: transforming growth factor-β1 induces prostaglandin E2 but not procollagen synthesis via a pertussis toxin-sensitive G-protein. Biochem J 1995;307:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-β isoforms on fibroblast cell cultures. Biochem J 1997;321:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 1995;4:1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriquez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-β in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 2001;58:1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, et al. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol 2004;165:1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol 2002;22:1631–1636. [DOI] [PubMed] [Google Scholar]
- 32.Hill MR, Papafili A, Booth H, Lawson P, Hubner M, Beynon H, Read C, Lindahl G, Marshall RP, McAnulty RJ, et al. Functional prostaglandin-endoperoxide synthase 2 polymorphism predicts poor outcome in sarcoidosis. Am J Respir Crit Care Med 2006;174:915–922. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins RG, Herrick SE, Meng QH, Kinnon C, Laurent GJ, McAnulty RJ, Hart SL. An integrin-targeted non-viral vector for pulmonary gene therapy. Gene Ther 2000;7:393–400. [DOI] [PubMed] [Google Scholar]
- 34.Moraes TJ, Chow CW, Downey GP. Proteases and lung injury. Crit Care Med 2003;31(4 Suppl):S189–S194. [DOI] [PubMed]
- 35.Nagai A, Aoshiba K, Ishihara Y, Inano H, Sakamoto K, Yamaguchi E, Kagawa J, Takizawa T. Administration of α1-proteinase inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am J Respir Crit Care Med 1992;145:651–656. [DOI] [PubMed] [Google Scholar]
- 36.Mitsuhashi H, Asano S, Nonaka T, Hamamura I, Masuda K-I, Kiyoki M. Administration of truncated secretory leukoprotease inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am J Respir Crit Care Med 1996;153:369–374. [DOI] [PubMed] [Google Scholar]
- 37.Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Effects of neutrophil elastase inhibitor on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 1997;156:260–265. [DOI] [PubMed] [Google Scholar]
- 38.Chua F, Dunsmore SE, Clingen PH, Mutsaers SE, Shapiro SD, Segal AW, Roes J, Laurent GJ. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am J Pathol 2007;170:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude O, Laurent GJ. Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J 1998;333:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers RC, Leoni P, Blanc-Brude OP, Wembridge DE, Laurent GJ. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem 2000;275:35584–35591. [DOI] [PubMed] [Google Scholar]
- 41.Dabbagh K, Chambers RC, Laurent GJ. From clot to collagen: coagulation peptides in interstitial lung disease. Eur Respir J 1998;11:1002–1005. [DOI] [PubMed] [Google Scholar]
- 42.Ruf W, Riewald M. Science review: role of coagulation protease cascades in sepsis. Crit Care 2003;7:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanc-Brude OP, Archer F, Leoni P, Derian C, Bolsover S, Laurent GJ, Chambers RC. Factor Xa stimulates fibroblast procollagen production, proliferation and calcium signaling via PAR1 activation. Exp Cell Res 2005;304:16–27. [DOI] [PubMed] [Google Scholar]
- 44.Howell DCJ, Goldsack NR, Marshall RP, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol 2001;159:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol 2005;166:1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkins RG, Su X, Su G, Scotton C, Camerer E, Laurent GJ, David G, Chambers R, Matthay M, Sheppard D. Ligation of protease-activated receptor 1 enhances α(v) β6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest 2006;116:1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurent GJ, Millward DJ. Protein turnover during skeletal muscle hypertrophy. Fed Proc 1980;39:42–47. [PubMed] [Google Scholar]
- 48.Eckes B, Krieg T. Regulation of connective tissue homeostasis in the skin by mechanical forces. Clin Exp Rheumatol 2004;22(3, Suppl 33):S73–S76. [PubMed] [Google Scholar]
- 49.Bishop JE, Butt RP, Laurent GJ. The role of mechanical force in the regulation of fibroblast function: implications for enhanced collagen deposition during pulmonary vascular remodelling. European Respiratory Review 1993;3:613–617. [Google Scholar]
- 50.Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol 2006;68:563–583. [DOI] [PubMed] [Google Scholar]
- 51.Torday JS, Rehan VK. Mechanotransduction determines the structure and function of lung and bone: a theoretical model for the pathophysiology of chronic disease. Cell Biochem Biophys 2003;37:235–246. [DOI] [PubMed] [Google Scholar]
- 52.Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports 2005;15:223–230. [DOI] [PubMed] [Google Scholar]
- 53.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 2004;7:709–718. [DOI] [PubMed] [Google Scholar]
- 54.Henson PM. Possible roles for apoptosis and apoptotic cell recognition in inflammation and fibrosis. Am J Respir Cell Mol Biol 2003;29(3, Suppl):S70–S76. [PubMed] [Google Scholar]
- 55.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol 2003;29:490–498. [DOI] [PubMed] [Google Scholar]
- 56.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 1998;275:L1192–L1199. [DOI] [PubMed] [Google Scholar]
- 57.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999;104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 2000;279:L143–L151. [DOI] [PubMed] [Google Scholar]
- 60.Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Transgenic modeling of transforming growth factor-β1: role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc 2006;3:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holgate ST, Lackie PM, Howarth PH, Roche WR, Puddicombe SM, Richter A, Wilson SJ, Holloway JW, Davies DE. Invited lecture: activation of the epithelial mesenchymal trophic unit in the pathogenesis of asthma. Int Arch Allergy Immunol 2001;124:253–258. [DOI] [PubMed] [Google Scholar]
- 62.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res 2002;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selman M, King TE Jr, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 64.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 2001;159:1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun 2007;353:104–108. [DOI] [PubMed] [Google Scholar]
- 69.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 2007;30:835–839. [DOI] [PubMed] [Google Scholar]
- 71.Das AM, Griswold DE, Molloy CJ, Protter AA, Laurent GJ. Fighting pulmonary fibrosis: new science brings new therapeutic opportunities. Drug Discov Today 2004;1:361–368. [Google Scholar]