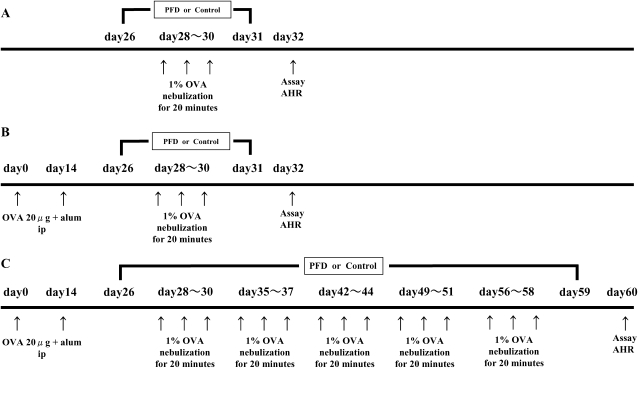
Figure 1.
Experimental protocols. (A) Mice were nonsensitized and received 3 consecutive days of aerosolized OVA challenge (nonsensitized/OVA). (B) Mice were sensitized by two intraperitoneal injections of OVA/alum and then received three consecutive days of aerosolized 1% OVA challenge (OVA/OVA). To evaluate the effect of pirfenidone on AHR and airway inflammation, mice received subcutaneous injections of pirfenidone (125, 250, or 500 mg/kg/d versus saline as vehicle) from Days 26 to 31. (C) To define the effect of pirfenidone on the airways after repeated OVA challenge, mice were exposed to aerosolized 1% OVA for 20 min 3 d per week for 4 wk (4 wk-OVA/OVA) and received subcutaneous injections of pirfenidone (500 mg/kg/d) from Days 26 to 59.