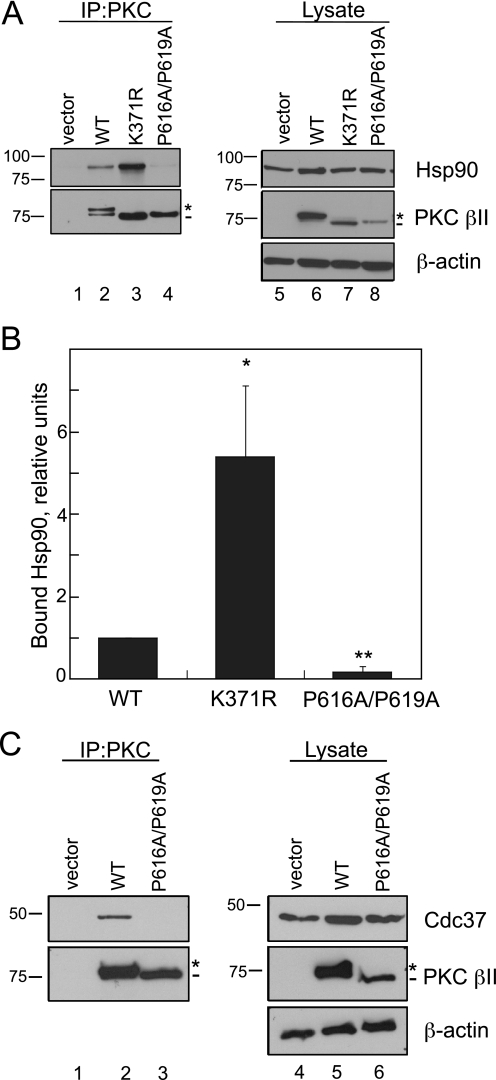
FIGURE 4.
Mutation of the PXXP motif in PKC βII decreases binding to the chaperones Hsp90 and Cdc37. A, COS7 cells were transfected with empty vector (lanes 1 and 5), WT PKCβII (lanes 2 and 6), PKCβII-K371R (lanes 3 and 7), or PKC βII-P616A/P619A (lanes 4 and 8). PKC was immunoprecipitated (IP) from detergent-solubilized lysates, and endogenous Hsp90 interaction was assessed by Western blot. Five percent of the detergent-solubilized lysates was analyzed to show the amount of Hsp90 and PKCβII present in the lysate. The asterisk denotes the position of fully phosphorylated PKC, and the dash denotes the position of unphosphorylated PKC. B, a graph representing densitometric analysis of the Western blots shown in A. Data represent the mean ± S.E. of six independent experiments. *, p < 0.05 versus WT, and **, p < 0.01 versus WT by Student's t test. C, COS7 cells were transfected with vector (lanes 1 and 4), WT PKC βII (lanes 2 and 5), or PKC βII-P616A/P619A (lanes 3 and 6). Endogenous Cdc37 interaction was assessed as described in A. The asterisk denotes the position of fully phosphorylated PKC, and the dash denotes the position of unphosphorylated PKC.