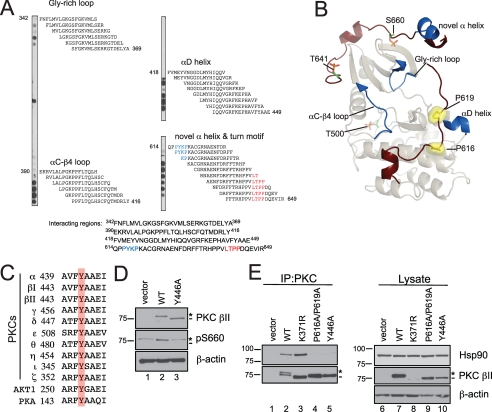
FIGURE 8.
Mutation of a conserved Tyr in the αE-helix of the catalytic domain of PKC βII mimics the defect of the PXXP mutant, PKC βII-P616A/P619A. A, peptide overlay of the catalytic domain of PKC βII with purified Hsp90α. Eighteen-residue peptides covering the catalytic domain (amino acids 342–673) of PKC βII and staggered by two amino acids were spotted onto a membrane and overlaid with 100 nm Hsp90α, and Hsp90 binding was detected with an anti-Hsp90 antibody. The residues highlighted in blue are the PXXP motif, and the residues highlighted in red are the residues delineating the turn motif phosphorylation site. B, ribbon diagram representation of the catalytic domain of PKC βII. A homology model of the PKC βII was built using the crystal structure of PKC θ (Protein Data Bank number: 1XJD) as the template. The C-terminal tail is highlighted in dark red with the Pro of the PXXP motif as yellow surface representations. The processing phosphorylation sites (activation loop (Thr-500), turn motif (Thr-641), and hydrophobic motif (Ser-660)) are shown as stick representations (orange and green). The regions identified in the peptide overlay are mapped onto the catalytic domain and are highlighted in dark blue. C, partial sequence alignment of part of the conserved αE-helix in the AGC kinases, the PKCs, Akt1, and PKA. A conserved Tyr is highlighted in red. D, COS7 cells were transiently transfected with empty vector (lane 1), WT PKC βII (lane 2), or PKC βII-Y446A (lane 3). Whole cell lysates were analyzed for total PKC (PKC βII), phosphorylated PKC (pSer-660), and total protein content (β-actin) by Western blotting. The asterisk denotes the position of phosphorylated PKC, and the dash denotes the position of unphosphorylated PKC. E, COS7 cells were transfected with empty vector (lanes 1 and 6), WT PKC βII (lanes 2 and 7), PKC βII-K371R (lanes 3 and 8), PKC βII-P616A/P619A (lanes 4 and 9), or PKC βII-Y446A (lanes 5 and 10). PKC was immunoprecipitated (IP) from detergent-solubilized lysates, and endogenous Hsp90 interaction was assessed by Western blot. Five percent of the detergent-solubilized lysates was analyzed to show the amount of Hsp90 and PKC βII present in the lysate. The asterisk denotes the position of fully phosphorylated PKC, and the dash denotes the position of unphosphorylated PKC. Data are representative of three independent experiments.