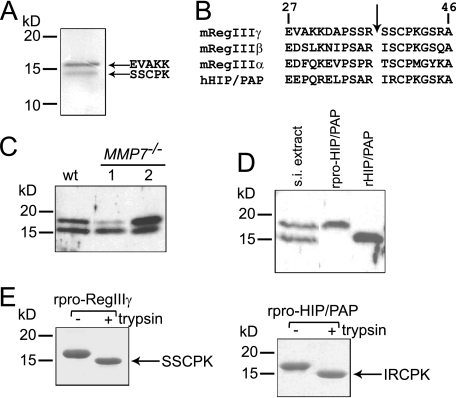
FIGURE 1.
RegIIIγ is proteolytically processed by trypsin in vivo. A, purification and N-terminal sequencing of endogenous mouse RegIIIγ reveals processing at the conserved N-terminal trypsin site. B, conserved canonical trypsin site (indicated by arrow) is present near the N terminus of mouse and human RegIII family members. Residue numbers are based on the deduced sequence which includes the signal peptide. Position 1 corresponds to the initiating methionine. C, MMP-7 is dispensable for RegIIIγ processing. 20 μg of protein extract from wild-type and MMP7–/– mice were immunoblotted with anti-RegIIIγ antibody. D, evidence for in vivo proteolytic processing of HIP/PAP. 20 μg of human intestinal protein extract was immunoblotted and probed with anti-RegIIIγ antiserum. Recombinant pro-HIP/PAP (rpro-HIP/PAP; with the N-terminal signal sequence replaced by methionine) and recombinant processed HIP/PAP (rHIP/PAP; with the N-terminal tryptic fragment replaced by methionine) were included for size comparison. s.i., small intestinal E, in vitro incubation of purified recombinant pro-RegIIIγ (rpro-RegIIIγ) and rpro-HIP/PAP with bovine trypsin results in quantitative cleavage at the conserved trypsin site to yield a homogeneous product. Proteins were digested with a 1:200 molar ratio of trypsin:lectin and were analyzed by SDS-PAGE. N-terminal sequencing verified cleavage at Arg37–Ser38 and Arg37–Ile38, respectively.