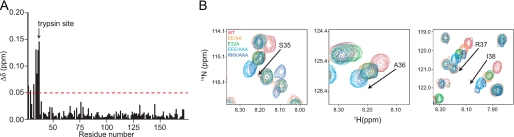
FIGURE 7.
Structural effects of activating pro-HIP/PAP mutations. A, chemical shift changes in the 15N/1H HSQC spectra of rpro-HIP/PAP and the activated mutant rpro-HIP/PAP-EEE/AAA are plotted as a function of residue number. Chemical shift changes >0.05 ppm are indicated with the red line. The trypsin cleavage site is indicated. B, superimposed 15N/1H HSQC spectra of 15N-labeled rpro-HIP/PAP and activating rpro-HIP/PAP mutations reveal colinear chemical shift perturbations among four residues surrounding the trypsin cleavage site at Arg37/Ser38. Arrows indicate the direction of larger chemical shift changes from wild-type and progression toward enhanced HIP/PAP killing activity.