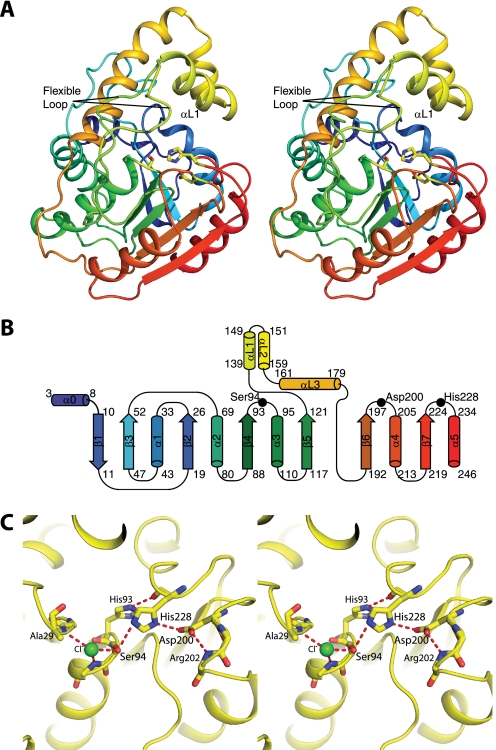
FIGURE 3.
Structure of RifR. A, stereodiagram of RifR TEII. In this ribbon diagram, the polypeptide is colored as a rainbow from blue at the N terminus to red at the C terminus. The lid domain is colored yellow. Active site triad residues (Ser94, Asp200, and His228) are shown as sticks. Two conformations (from different crystal forms) are shown for the flexible linker region between strand β5 and the lid domain. B, topology of RifR. C, stereodiagram of the active site. The catalytic triad (Ser94, Asp200, and His228) and surrounding residues are shown as sticks. A chloride ion (green) occupies the oxyanion hole formed by backbone amides of residues Ala29 and Met95 (side chain not shown). The atomic colors are used in A and C for stick figures with yellow as carbon, red as oxygen, blue as nitrogen, and green as chlorine.