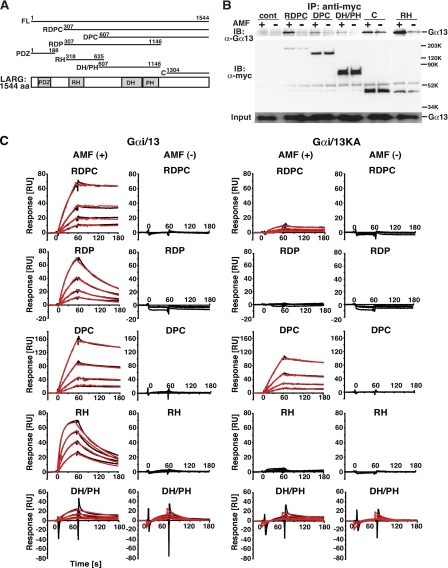
FIGURE 1.
Direct interaction of LARG with Gα13 through its RH domain, DH/PH domains, and C-terminal region. A, schematic representation of LARG and its deletion constructs. The amino acid numbers encoded in each constructs are listed. PDZ, PDZ domain; RH, RGS homology domain; DH, Dbl homology domain; PH, pleckstrin homology domain. A full-length, RDPC, DPC, RDP, PDZ, RH, DH/PH, or C-terminal region of LARG is referred to as LARG-FL, -RDPC, -DPC, -RDP, -PDZ, -RH, -DH/PH, or -C, respectively. B, the binding of various LARG proteins to Gα13 in COS1 cells. COS1 cells were co-transfected with Gα13WT (0.5 μg) and the indicated myc-tagged LARG constructs: RDPC (5 μg), DPC (4 μg), DH/PH (3 μg), C(4 μg), and RH (5 μg). The LARG proteins were immunoprecipitated by anti-Myc antibody from cell lysates in the presence or absence of AMF. Immunoprecipitates were separated by SDS-PAGE, followed by Western blotting using anti-Gα13 antibody or anti-Myc antibody. C, kinetics of binding of LARG to Gαi/13 or Gαi/13KA immobilized on the SPR biosensor. Gαi/13 and Gαi/13KA proteins were immobilized on parallel channels of the Biacore sensor chip CM5 as described under “Experimental Procedures.” The association phase of the reaction between serially diluted LARG fragments and Gαi/13 was 2 min, and the dissociation phase was 1 min at 15 °C. The interactions were measured using Biacore 3000. Black lines show the experimental data. Red lines show fitting data analyzed as simultaneous ka/kd, 1:1 binding, and global fitting using the BIAevaluation program. In the absence of AMF, kinetic analyses were not performed when the responses were <1/10 of those with Gαi/13 in the presence of AMF. The concentrations of proteins were: FL, 1.1–17.5 nm; RDPC, 4.4–70 nm; RDP, 1.1–17.5 nm; DPC, 37.5–600 nm; RH, 8.8–140 nm; and DH/PH, 78.1 nm to 1.25 μm.