Abstract
Blastocladiella emersonii is an aquatic fungus of the Chytridiomycete class. During germination, the zoospore, a motile nongrowing cell, goes through a cascade of morphological changes that culminates with its differentiation into the germling cell, capable of coenocytic vegetative growth. Transcriptome analyses of B. emersonii cells were carried out during germination induced under various environmental conditions. Microarray data analyzing 3,563 distinct B. emersonii genes revealed that 26% of them are differentially expressed during germination in nutrient medium at at least one of the time points investigated. Over 500 genes are upregulated during the time course of germination under those conditions, most being related to cell growth, including genes involved in protein biosynthesis, DNA transcription, energetic metabolism, carbohydrate and oligopeptide transport, and cell cycle control. On the other hand, several transcripts stored in the zoospores are downregulated during germination in nutrient medium, such as genes involved in signal transduction, amino acid transport, and chromosome organization. In addition, germination induced in the presence of nutrients was compared with that triggered either by adenine or potassium ions in inorganic salt solution. Several genes involved in cell growth, induced during germination in nutrient medium, do not show increased expression when B. emersonii zoospores germinate in inorganic solution, suggesting that nutrients exert a positive effect on gene transcription. The transcriptome data also revealed that most genes involved in cell signaling show the same expression pattern irrespective of the initial germination stimulus.
Blastocladiella emersonii is a saprobic aquatic fungus, belonging to the Chytridiomycete class, located at the base of the fungal phylogenetic tree. Its life cycle is characterized by two distinct stages of cell differentiation: germination and sporulation. Under adverse nutritional conditions, the fungus stops growing and enters the sporulation stage, which after a series of morphological changes culminates with the production and release of the zoospores, motile cells that are important for the survival and dispersal of the organism. The zoospore is a wall-less cell that neither grows nor divides and can swim for hours using its endogenous reserves. In the presence of appropriate stimuli the zoospore germinates, undergoing a series of drastic morphological and biochemical changes. In the first 15 min of germination, the zoospore retracts its single polar flagellum and forms a thin cell wall of chitin, becoming a round cell. By this time, a cellular efflux of calcium (8), mobilization of cellular glycogen (45), and a decrease in lipid contents (34) have occurred. These events do not require concomitant translation or transcription and are directed by proteins and mRNAs stored in the zoospore (20, 23, 30, 37, 38). Next, the round cell converts into a vegetative cell, the germling cell, with the formation of a germ tube that elongates and begins to branch at approximately 60 min, giving rise to a rhizoidal system through which nutrients are absorbed (41). Conversion of the round cell into a vegetative cell is accompanied by a large stimulation of protein synthesis. This increase in protein synthesis seems to be mediated by the mobilization of stored mRNA into polyribosomes between 15 and 30 min after induction of germination (10, 32).
Germination is a controlled process that responds to environmental stimuli. In B. emersonii, germination can be induced either by the addition of nutrient medium or in an inorganic solution containing 50 mM KCl (35). Van Brunt and Harold (44) suggested that potassium ions trigger encystment by causing membrane depolarization. Many other inducers have been identified, such as cyclic AMP (cAMP), cyclic GMP (cGMP), and N-(alkylated) xanthines (adenine and adenosine), all of them inducing germination with a high degree of synchrony (9, 11, 31). Soon after germination is triggered, a transient increase in intracellular cAMP levels is detected (43), and a concomitant activation of the cAMP-dependent protein kinase is observed (1). All the enzymes of the cAMP metabolism are developmentally regulated in the fungus, with very low activities during exponential growth and increasing activities during sporulation that reach maximal levels in the zoospores (6, 15, 24). Together these data suggest an important role for cAMP and protein phosphorylation in the germination process.
Calcium, another important second messenger in signaling pathways of eukaryotic cells, was also found to play a role during B. emersonii germination. As mentioned above, during zoospore encystment a large efflux of calcium is observed. In addition, lanthanum, which blocks the uptake and efflux of Ca2+, inhibits germination completely when added at the time of induction (8). Calcium was also found to be both necessary and sufficient for sporulation of B. emersonii, and low levels of Ca2+ were observed to enhance the stability of zoospores (2, 35, 36). Calmodulin (CaM), a small acidic protein whose primary role is to serve as an intracellular Ca2+ receptor modulating the activity of numerous intracellular proteins involved in diverse signaling pathways (49), is found in B. emersonii zoospores (7, 33). Furthermore, pharmacological agents known to antagonize CaM action were shown to inhibit germination if added at the time of induction, indicating an important role for calcium and CaM during this morphogenetic transition (33). These observations indicate that the molecular mechanisms involved in triggering germination involve multiple sensors and pathways, each one possibly sensitive to a certain environmental stimulus, working in a particular combination.
To complement the studies concerning the morphological and biochemical events associated with B. emersonii germination, it is important to establish a comprehensive evaluation of the genes that have their expression modulated during this process. In order to achieve this purpose, we constructed a 9,216-element array containing 3,563 distinct expressed sequence tag sequences, obtained from cDNA libraries constructed with RNA from cells at different stages of the life cycle of the fungus (28) and from cells under heat or cadmium stress (5). The microarrays were used to study the global changes in transcript levels in cells isolated at different times after induction of germination in nutrient medium. More than 900 genes were differentially expressed during germination in nutrient medium at at least one of the time points analyzed, corresponding to 26% of the genes in the microarrays. We also analyzed the differences between germination triggered in nutrient medium and in inorganic solution where the effective inducers were either adenine or potassium ions. Analysis of these data allowed us to identify specific genes and gene sets whose expression seems to be related to the biochemical and morphological changes associated with B. emersonii germination.
MATERIALS AND METHODS
Culture conditions and induction of germination.
Cultures of B. emersonii were maintained on plates containing 0.13% peptone, 0.13% yeast extract, 0.3% glucose, and 1.5% agar. For RNA extraction, zoospores were inoculated (3.0 × 105 cells/ml) in defined DM3 liquid medium (24) and grown for 16 h at 17°C with agitation (150 rpm). After this period of growth, vegetative cells were collected by filtration through a Nitex cloth, rinsed, and resuspended in sporulation solution (SE; 1 mM Tris-maleate buffer, pH 6.8, containing 1 mM CaCl2) at a density of 1.0 × 106 cells/ml. Vegetative cells were incubated at 27°C with agitation until the zoospores were completely released (3.5 to 4.0 h). The new zoospores were separated from the empty vegetative cells by filtration and collected by centrifugation (1,000 × g at 4°C), suspended in 1 ml of SE, and either frozen on liquid nitrogen for total RNA extraction or inoculated into a proper solution for germination.
The solutions used to induce germination were defined DM3 medium; germination solution (1 mM Tris-maleate buffer, pH 6.8, containing 1 mM CaCl2, 10 mM MgCl2, and 50 mM KCl); and germination solution without KCl, containing 2.5 mM adenine. Zoospores were inoculated in the appropriate solution at a density of 1.0 × 106 cells/ml and incubated at 27°C with agitation. The progress and synchrony of germination were monitored by taking samples at different times and examining cell types under a light microscope. Cells were collected by vacuum filtration at different times of germination and frozen in liquid nitrogen for total RNA extraction.
RNA isolation.
Total RNA was isolated using Trizol LS (Life Technologies), and the integrity of the RNA was checked through agarose-2.2 M formaldehyde gel electrophoresis, followed by ethidium bromide staining and RNA visualization under UV light. RNA samples were isolated from zoospores and from germinating cells at 30, 60, and 90 min after induction of germination in DM3 medium and at 30 and 60 min after induction of germination in either germination solution or germination solution without KCl and with 2.5 mM adenine.
PCR amplification and array printing.
Microarray chips were designed to contain 3,563 distinct expressed sequence tag sequences obtained from cDNA libraries constructed with RNA from cells at different stages of the life cycle of the fungus (28) and cells exposed to heat shock or cadmium stress (5). B. emersonii plasmid clones were amplified in 100-μl PCR mixtures (40 cycles, with annealing at 51°C) directly from bacterial clones in culture, using T7 and SP6 primers. Samples were visualized on 1% agarose gels to inspect PCR amplification quality and quantity. PCR products were purified by filtration using 96-well Millipore Multiscreen filter plates and eluted in 10 mM Tris-HCl solution at pH 8.0. Purified PCR products were mixed with an equal volume of dimethyl sulfoxide in 384-well V-bottom plates. Microarrays were constructed by arraying cDNA fragments on dimethyl sulfoxide-optimized, metal-coated glass slides (type 7 star; Amersham Biosciences) using a Generation III microarray spotter (Molecular Dynamics/Amersham Pharmacia Biotech). Each cDNA fragment was spotted at least in duplicate (i.e., technical replicates). Following printing, the slides were allowed to dry and the spotted DNA was bound to the slides by UV cross-linking (50 mJ).
All DNA fragments spotted on the slides were previously sequenced in order to confirm their putative identity. About 87% of the fragments had their identity validated, and the others were reannotated after BlastX against a local database constructed with sequences from Swiss-Prot and TrEMBL (http://www.expasy.org) according to Gene Ontology (GO) terms (http://www.geneontology.org). All the best hits identified had E-values smaller than 10−6.
Probe preparation and hybridization and data analysis.
Ten micrograms of total RNA were reverse transcribed and labeled by using a CyScribe post-labeling kit (GE Healthcare) according to the manufacturer's instructions. Briefly, the RNA was reverse transcribed using the enzyme CyScript in the presence of modified amino allyl-dUTP, an optimized nucleotide mix, buffer, dithiothreitol, and random primers. The resulting amine-modified cDNA was then chemically labeled in the amino allyl group by using CyDye N-hydroxysuccinimide esters in 0.1 M sodium bicarbonate (pH 9.0). The products of the labeling reactions were purified in Millipore multiscreen filtering plates to remove unincorporated labeled nucleotides.
Microarrays were cohybridized with the fluorescence-labeled probes. Hybridizations were performed overnight at 42°C. The slides were then washed in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.2% sodium dodecyl sulfate (10 min at 55°C), twice in 0.1×SSC and 0.2% sodium dodecyl sulfate (10 min at 55°C), and in 0.1× SSC (1 min at room temperature). The slides were rinsed briefly in Milli-Q water and dried under a nitrogen stream. The hybridizations were performed as displayed in Table 1. Each experimental condition was analyzed with three independent biological experiments. Since each slide carried two replicates of the arrayed genes, a total of six intensity readings were generated for each gene in the microarray. Slides were scanned with a Generation III ScannerTM (Molecular Dynamics), with the photomultiplier tube adjusted to 700 for both channels. The fluorescence mean intensity and surrounding median background from each spot were obtained with ArrayVision, version 6.0 (Imaging Research, Inc.). Data from clones that generated poor-quality PCR fragments (no amplification or unspecific bands) or poor-quality spots (visually inspected) were excluded. Normalization was carried out by LOWESS fitting on an M-versus-S plot, where M is the fluorescence log ratio of the test sample relative to the control condition [M = log2(test/control)] and S is the log-mean fluorescence intensity {S = log2[(1/2)test + (1/2)control]} (19).
TABLE 1.
Scheme for comparison of germination in nutrient medium with germination induced in the presence of either adenine or potassium
| Source of RNA for competitive hybridization | Germination time point(s) (min) at which cells were tested
|
|
|---|---|---|
| Reference RNA | Test RNA | |
| Germination induced in nutrient medium DM3 | Zoospores; time 0 of germination | 30, 60, 90 |
| Germination induced by either adenine or potassium vs reference | ||
| DM3 induction | 30, 60 | |
| Adenine induction | 30, 60 | |
| Potassium induction | 30, 60 | |
Determination of differentially expressed genes.
We used intensity-dependent cutoff values for classifying a gene as differentially expressed based on the results of self-self hybridization experiments (46). In this type of hybridization, the same cDNA sample is labeled independently with both Cy3 and Cy5 dyes to estimate the experimental noise. Two distinct self-self experiments were performed, one with RNA from zoospores and another with RNA from germinating cells. The HTself program, available on the web (http://blasto.iq.usp.br/∼rvencio/HTself), was used to determine the intensity-dependent cutoff curves. These curves delimit the boundaries of intrinsic experimental noise and, therefore, genes which do not show statistically significant variation in their expression levels. Using these intensity-dependent cutoff values, we were able to determine which genes were differentially expressed during germination. Genes that presented at least 80% of replicates with expression ratios above or below the cutoff limits determined by self-self hybridization experiments were considered to be upregulated or downregulated, respectively.
Clustering analysis and determination of overrepresented functional gene categories in each group.
Differentially expressed genes presenting the complete time course profile (0, 30, 60, and 90 min of germination in nutrient medium) were clustered in 10 groups according to their expression patterns by using the K-means algorithm implemented in the SpotWhatR software (19). To characterize each K-means group based on functional gene categories, we measured the level of statistical association between “being in a given group” and “belonging to a functional category” by using the BayGO method (47). We considered that a gene category was overrepresented if the value of the statistical significance was smaller than 0.05.
Validation of microarray data by quantitative real-time RT-PCR.
To evaluate the reliability of the array-based data, seven genes were randomly selected and their expression levels analyzed by quantitative real-time RT-PCR (qRT-PCR). Appropriate primers were designed by using Primer Express 2.0 software (Applied Biosystems). qRT-PCR experiments were performed using GeneAmp 5700 sequence detection system (Applied Biosystems) equipment and a platinum SYBR green qPCR SuperMix-UDG kit (Invitrogen). The thermocycling conditions comprised an initial step at 50°C for 2 min, followed by 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. The specificity of the amplified products was evaluated by analysis of the dissociation curves generated by the equipment. Two independent RNA samples were used for each gene analyzed. The gene encoding a putative mitochondrial RNA helicase-like protein was used as the calibrator gene in all experiments. The determination of the expression ratios was carried out by using the threshold cycle (ΔΔCT) method described by Livak and Schmittgen (22).
Microarray data accession number.
The microarray data discussed in this work have been deposited in NCBI's Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE12883 (http://www.ncbi.nih.gov/geo/query/acc.cgi?acc=GSE12883).
RESULTS AND DISCUSSION
Global gene expression analysis.
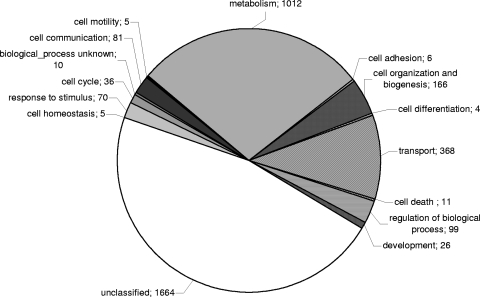
To investigate global changes in gene expression during B. emersonii's germination process, we constructed cDNA microarrays containing 3,563 putative unique genes, which were identified in 12 distinct cDNA libraries prepared using RNA isolated from cells at different stages of the life cycle of the fungus (28) and from cells exposed to heat shock or cadmium stress (5). The PCR-amplified cDNAs spotted on the arrays were resequenced in order to confirm their identity and were classified according to GO terms as shown in Fig. 1.
FIG. 1.
Functional classification of the B. emersonii genes spotted on the microarrays. The numbers of B. emersonii genes classified as involved in different biological processes according to the terms of the Gene Ontology Consortium are shown.
The genes differentially expressed during germination were investigated in time course experiments, using RNA samples isolated from cells collected 30, 60, and 90 min after inoculation of zoospores in nutrient medium DM3. The reference sample was zoospore RNA, which was considered the time zero of germination. Competitive microarray hybridizations were carried out with RNA isolated from three independent biological experiments. We also compared germination in nutrient medium with germination induced in inorganic salt solution, triggered either by adenine or potassium ions. In these experiments we considered RNA from cells germinated in nutrient medium to be the reference sample. The different competitive hybridizations carried out are described in Table 1. A particular gene was classified as differentially expressed during germination in comparison with its expression in the reference sample if at least 80% of the replicates were outside the credibility intervals defined in self-self hybridization experiments by using the software HTself (46), using at least three valid replicates, as described in Materials and Methods.
Gene expression profiles during germination in nutrient medium.
Analysis of competitive hybridization data according to the criteria described above revealed a total of 535 genes upregulated and 389 genes downregulated at at least one of the time points analyzed when cells germinating in nutrient medium were compared to zoospores. Differentially expressed genes were then clustered according to their expression profiles into 10 groups by using the K-means algorithm. Gene clusters were classified into GO functional categories, and a search for overrepresented categories in each group was carried out by using BayGO software (47), as described in Materials and Methods. Figure 2 and Table 2 show the expression profiles obtained through K-means clustering and the corresponding overrepresented gene categories, respectively.
FIG. 2.
K-means clustering with 10 groups using the complete profiles of differentially expressed genes. The y axis shows the log2 expression ratio of the normalized values, and the x axis shows the time after induction of germination in nutrient medium. The overrepresented gene categories for each cluster are shown in Table 2.
TABLE 2.
Overrepresented gene categories for K-means clustersa
| Group | Overrepresented gene categories |
|---|---|
| K1 | Protein biosynthesis, small-GTPase-mediated signal transduction, calcium ion transport, protein folding |
| K2 | rRNA processing, transcription, ribosome biogenesis, purine nucleotide biosynthesis, cell adhesion, amino acid metabolism |
| K3 | Protein biosynthesis, glycolysis |
| K4 | Translation initiation factor activity, protein biosynthesis, carbohydrate transport, oligopeptide transport, cell cycle |
| K5 | Tricarboxylic acid cycle, glycolysis, fatty acid biosynthesis, anion transport, GTP biosynthesis, UTP biosynthesis, protein nucleus import |
| K6 | Chitin synthase activity, proton transport |
| K7 | Signal transduction, actin-binding activity |
| K8 | Ion channel activity |
| K9 | Amino acid transport, intracellular protein transport, protein serine/threonine kinase activity, calcium ion-binding activity, signal transducer activity |
| K10 | Chromosome organization and biogenesis |
A functional category was considered overrepresented if its statistical association with its presence in the cluster was significant (P < 0.05) (see Fig. 2).
To validate the expression profiles obtained in microarray experiments, qRT-PCR assays were performed for seven randomly selected genes using cell samples from two independent biological time course experiments of germination in nutrient medium. To normalize the data, the gene encoding a mitochondrial RNA helicase-like protein was used, as its expression showed no change in the microarray experiments under all conditions tested. Figure 3 shows the comparison of the results from microarrays and qRT-PCR experiments for time points 30, 60, and 90 min of germination in nutrient medium, using zoospore RNA (time zero) as the reference. All results for genes analyzed by qRT-PCR confirmed the expression profile from the microarrays.
FIG. 3.
Expression levels of seven selected genes during germination in nutrient medium, evaluated by real-time RT-PCR (open triangles) and microarray experiments (black squares). M is the log2 expression ratio of the normalized values. The results are the median values from independent biological samples. Vertical bars represent standard deviations. CoA, coenzyme A.
Genes upregulated during germination in nutrient medium.
Genes classified as upregulated during B. emersonii germination were clustered in groups K1 to K5, and the categories overrepresented in these groups according to BayGO are shown in Table 2. The complete list of genes in groups K1 to K5 and their expression ratios are found in Table S1 in the supplemental material. The main biological processes observed among the upregulated genes are those necessary for cell growth and maintenance, including gene transcription, protein biosynthesis, energy metabolism, nutrient transport, and cell cycle control. These results are in agreement with the events that are common to the germination processes described for other fungi (3, 27, 48).
The best-represented functional category observed among the upregulated genes is protein biosynthesis, which includes genes encoding ribosomal proteins and translation initiation and elongation factors (K1, K3, and K4), as well as genes related to protein folding (K2) and ribosome biogenesis (K1). Altogether, 56 genes involved in protein biosynthesis were upregulated during germination in nutrient medium at the time points analyzed. The induction of B. emersonii genes involved in ribosome biogenesis during germination in nutrient medium coincides temporally with the increase of polyribosome formation previously observed (10, 30) and is probably related to the sudden increase in the rate of protein synthesis that occurs early in germination, using the preformed mRNAs stored in the zoospores (30). The ability of fungal spores to store prepackaged mRNA has also been described for Aspergillus nidulans and Neurospora crassa (14, 27). The conidia of these fungi show high levels of free ribosomes that associate with presynthesized RNAs to form polyribosomes in the presence of a carbon source, and the stored mRNAs are primed for rapid activation and translation.
Among the upregulated genes, we also detected genes involved in carbohydrate and oligopeptide transport, responsible for nutrient uptake from the environment (K4). This group also includes genes related to cell cycle regulation that control cell entry into S phase and mitosis. As expected, several genes involved in the energy metabolism pathway were also induced during the first 90 min of germination in nutrient medium: glycolysis (K3 and K5), tricarboxylic acid cycle (K5), and fatty acid biosynthesis (K5) genes. Some of these genes encode key enzymes in energetic metabolism, such as pyruvate kinase, citrate synthase, and isocitrate dehydrogenase (see Table S1 in the supplemental material).
As depicted in Fig. 2, genes in groups K4 and K5 show the highest induction levels. Genes in these groups play a role in important biological processes, such as protein biosynthesis, nutrient uptake, and energetic metabolism, and are among the 20 most-highly induced genes (see Table S1 in the supplemental material). In addition, a large number of genes (147 genes) with no putative function assigned were classified as upregulated during germination in nutrient medium, suggesting a role in the germination process and constituting a first characterization of these genes (see Table S1 in the supplemental material).
Several recent microarray studies have also investigated differentially expressed genes during germination in fungi, including Trichophyton rubrum (21), Neurospora crassa (16), and Ustilago maydis (50). Most of the genes upregulated in these organisms correspond to the same functional categories observed during B. emersonii germination, for instance, protein biosynthesis and primary metabolism involved in energy production of the cell.
Genes downregulated during germination in nutrient medium.
The early morphological events of B. emersonii germination have been shown to occur in the absence of transcription, and data indicate that the mRNAs necessary to begin the germination process are probably synthesized in the final stages of sporulation and stored in the zoospores (30). Among these transcripts we would expect to find those present in the zoospores and downregulated during germination in nutrient medium. The expression profiles of genes clustered in groups K6 to K10 encompass such transcripts. The functional categories overrepresented among the genes in groups K6 to K10 according to BayGO are shown in Table 2, and the complete set of genes are listed in Table S2 in the supplemental material. Some of the overrepresented functional categories observed among these genes are discussed bellow.
Signal transduction.
Zoospores must sense the environment in order to recognize the appropriate conditions to germinate. So, it is imperative that they possess the necessary means to sense and respond to changes in the environment, choosing the best moment to germinate. In B. emersonii zoospores, we detected transcripts encoding proteins involved in different signaling pathways (groups K7 and K9) (see Table S2 in the supplemental material): a Gsα subunit, a cGMP phosphodiesterase, a soluble guanylate cyclase (its transcript levels do not change during germination in nutrient medium but increase during germination induced by adenine [data not shown]), proteins of the mitogen-activated protein kinase pathway, phosphatases protein phosphatase 2A (PP2A) and calcineurin (a phosphatase that is stimulated by CaM), a Ras GTPase, and also the catalytic and regulatory subunits of the cAMP-dependent protein kinase A (PKA). These data corroborate previous biochemical studies indicating that germination in B. emersonii seems to be controlled by more than one signaling pathway (see the introduction).
The regulatory and catalytic subunits of the PKA of B. emersonii were previously shown to present their highest levels in zoospores, decreasing to almost undetectable levels during germination (25), which is consistent with the transcript profiles of the corresponding genes reported here. cGMP phosphodiesterase activity was also determined throughout the life cycle of the fungus (42) and shown to present the same pattern of variation observed for the PKA activity. Thus, the expression profile for the cGMP phosphodiesterase transcript found here is in agreement with the changes in enzyme activity during germination.
PP2A affects a variety of biological processes in the cell, such as transcription, cell cycle progression, and cellular morphogenesis (17). This phosphatase is also involved in many aspects of cellular function, including the regulation of metabolic enzymes and proteins involved in signal transduction (26). PP2A has been previously investigated for B. emersonii (4). The authors demonstrated that its activity is developmentally regulated, increasing during sporulation and reaching maximum levels in zoospores. They also showed that the protein phosphatase inhibitor okadaic acid induces encystment but inhibits germ tube formation. Microarray data revealed that the transcript encoding the regulatory subunit of PP2A is present in zoospores and is severely downregulated during germination in nutrient medium. In addition, the transcript encoding the PP2A inhibitor is highly induced during germination. Thus, in zoospores, where there is an arrest in the cell cycle, PP2A regulatory subunit transcript levels are high and PP2A inhibitor transcript levels are low, whereas during germination, when the cell cycle is resumed, the opposite behavior is observed. These results agree with the hypothesis that this PP2A negatively controls B. emersonii germination. Interestingly, previous work has shown that in fission yeast, PP2A negatively regulates entry into mitosis (18).
Amino acid transporters.
Several transcripts encoding amino acid transporters are stored in B. emersonii zoospores and are downregulated during germination in nutrient medium (K9) (see Table S2 in the supplemental material). It is known that nutrients, besides their role in metabolism, also exert regulatory effects that seem to be mediated by nutrient sensors present in the cell membrane. In Saccharomyces cerevisiae, for instance, the Gap1 protein is an amino acid permease that acts not only as a transporter, but also as an amino acid sensor. The activity of Gap1 is enhanced in cells in the absence of nitrogen, but when cells are supplemented with ammonium, l-glutamine, or l-glutamate, this protein is inactivated both at the transcriptional and posttranscriptional levels (13, 39, 40). The downregulation of these amino acid transporter genes in germination triggered by nutrient medium suggests a similar role for these genes in B. emersonii.
Histones and histone deacetylase.
Another interesting observation is a significant decrease in the expression levels of genes encoding histones during B. emersonii germination in nutrient medium (K10) (see Table S2 in the supplemental material). Furthermore, the transcript for a putative histone deacetylase is present in zoospores and downregulated during germination in nutrient medium, when unblocking of transcription occurs (see Table S2 in the supplemental material). The removal of acetyl groups from histone tails, an event catalyzed by histone deacetylases, is a means of implementing gene silencing (12). The presence of histone deacetylase transcripts in the zoospores is a hint that chromatin structure could be responsible for the inhibition of replication and transcription observed in these cells. So, the repression of the genes encoding histones and a histone deacetylase during germination in nutrient medium could be required to resume DNA replication and transcription.
In addition, a large number of genes (a total of 192) with no putative function assigned were also downregulated during germination (see Table S2 in the supplemental material), thirteen of them being among the 20 most highly repressed genes (see Table S2 in the supplemental material).
Comparison of germination induced in nutrient medium and in inorganic solution.
To investigate if different genetic pathways are utilized during germination occurring in the absence of nutrients, we compared germination in growth medium with that induced in inorganic salt solution, triggered either by adenine or potassium. Morphologically, the early events of germination during the first 60 min, such as the retraction of the flagellum, synthesis of the cell wall, germ tube formation, and initial branching, are the same under the three conditions tested (data not shown). However, 90 min after induction in the absence of nutrients, cells fail to grow and begin to show dark spots in the cytoplasm (not shown).
Comparison of global gene expression profiles of cells germinating in nutrient medium with those of cells germinating in inorganic salt solution was carried out by using competitive microarray hybridizations. In these experiments, we used RNA obtained from cells isolated 30 and 60 min after induction of germination, according to the hybridization scheme presented in Table 1, rows 2 to 5.
A high number of differentially expressed genes were observed during germination triggered by adenine or potassium relative to expression during germination in the presence of nutrients. Figure 4 shows the number of genes upregulated or downregulated in each case. These data suggest that the control of expression of a large number of genes during germination is responsive to the presence of nutrients in the medium.
FIG. 4.
Numbers of genes differentially expressed when germination induced by adenine (A) or potassium (K) is compared with germination in nutrient medium. Numbers in overlaps represent genes differentially expressed with both inducers.
It is noteworthy that several genes involved in cell signaling pathways (Table 3) are not differentially expressed when we compare germination induced in nutrient medium with germination triggered in inorganic salt solution. These results suggest that many cell signaling pathways are activated independently of the initial stimulus triggering the germination process.
TABLE 3.
Genes involved in cell signaling presenting the same expression profile irrespective of the inducer of germination
| Clone ID | Best alignment (Swiss-Prot) | GO description |
|---|---|---|
| BeE60H30H04 | O93887; G protein beta subunit GPB1 | G protein-coupled receptor protein signaling pathway |
| BeG120N11H04 | Q05425; guanine nucleotide-binding protein alpha-1 subunit | G protein-coupled receptor protein signaling pathway |
| BeE60N16D02a | Q54PH7; G protein subunit alpha 8 | G protein-coupled receptor protein signaling pathway |
| BeZSPN08H03 | Q5KPS8; heterotrimeric G protein alpha subunit B, putative | G protein-coupled receptor protein signaling pathway |
| BeE120N26D09 | Q68EF8; RapGEF-like 1 protein (fragment) | G protein-coupled receptor protein signaling pathway |
| BeE60H26A07 | Q870G5; guanine nucleotide-binding protein gamma subunit | G protein-coupled receptor protein signaling pathway |
| BeG60N08H03a | Q8J0B8; G protein alpha subunit | G protein-coupled receptor protein signaling pathway |
| BeE120N28E07a | Q9HFN1; G protein alpha subunit (fragment) | G protein-coupled receptor protein signaling pathway |
| BeE120N18F01a | Q86WN6; phosphodiesterase PDE9A13 | cAMP/cGMP-mediated signaling |
| BeE60N08A04 | Q3ZLB7; ASP | cAMP-dependent protein kinase, regulator activity |
| BeE120N30B12 | P31320; cAMP-dependent protein kinase regulatory subunit | cAMP-dependent protein kinase, regulator activity |
| BeZSPN16D09a | Q12741; cAMP-dependent protein kinase catalytic subunit | Protein serine/threonine kinase activity |
| BeE60N11G06a | Q05116; dual specificity MAP | Protein serine/threonine kinase activity |
| BeE60N03C06a | Q9HDE1; calcineurin B regulatory subunit | Calmodulin-stimulated protein phosphatase |
| BeG30N18A11 | Q15269; periodic tryptophan protein 2 homolog | Signal transduction |
| BeE60C09B03 | Q4KIT4; methyl-accepting chemotaxis protein | Signal transduction/chemotaxis |
| BeE120N04B04a | Q9Y4G8; Rap guanine nucleotide exchange factor 2 | cAMP-mediated signaling/mitogen-activated protein kinase kinase kinase cascade/small-GTPase-mediated signal transduction |
| BeE60H31A02 | Q4DZ75; putative small-GTP-binding protein Rab28 | Small-GTPase-mediated signal transduction |
| BeE30N10H08 | Q4P7T0; YPT1_NEUCR GTP-binding protein ypt1 | Small-GTPase-mediated signal transduction |
| BeE90N24C02 | Q4PB75; hypothetical protein | Small-GTPase-mediated signal transduction |
| BeZSPN11G01 | Q4PG14; hypothetical protein | Small-GTPase-mediated signal transduction |
| BeE120N32A08 | Q4WGK7; septin B | Small-GTPase-mediated signal transduction |
| BeZSPN17H07 | Q4WZP3; cell division control protein Cdc25, putative | Small-GTPase-mediated signal transduction |
| BeE30N13H07 | Q5K7V1; putative GTPase | Small-GTPase-mediated signal transduction |
| BeE60H32H12 | Q7Q7M3; ENSANGP00000020929 | Small-GTPase-mediated signal transduction |
| BeE90N21E01 | Q7RVG3; RAS-related protein RAB1BV | Small-GTPase-mediated signal transduction |
| BeG30N14G03 | Q7T0S9; MGC69017 protein | Small-GTPase-mediated signal transduction |
| BeE120N24H03 | Q86F33; clone ZZD1150 mRNA sequence | Small-GTPase-mediated signal transduction |
| BeE120N26G04 | Q86G47; nucleotide exchange factor RasGEF Q | Small-GTPase-mediated signal transduction |
| BeE60N03A04 | Q8K2P9; Rabl2a protein | Small-GTPase-mediated signal transduction |
| BeE60N07H08 | Q8T367; small G protein | Small-GTPase-mediated signal transduction |
| BeZSPN15D02 | Q9PW31; Rac GTPase | Small-GTPase-mediated signal transduction |
| BeE30N04H05 | Q9Y2Y0; Arf-like 2-binding protein BART1 | Small-GTPase regulatory/interacting protein activity |
| BeE60C35C07 | Q7WF10; phosphate regulon sensor protein | Two-component sensor molecule activity |
| BeE60C18E02 | Q3KBH6; p-aminosalicylic acid/PAC sensor hybrid histidine kinase | Two-component signal transduction system (phosphorelay) |
| BeE60C36F03 | Q4KAJ4; sensor histidine kinase/response regulator | Two-component signal transduction system (phosphorelay) |
| BeE60C14D12 | Q4ZP96; PAS domain | Two-component signal transduction system (phosphorelay) |
Transcript is downregulated during germination in nutrient medium.
Microarray expression data obtained in germination assays triggered by adenine (30 and 60 min) were also validated by using qRT-PCR experiments. In this case the RNA used as the reference was that from cells at the corresponding time points of germination induced in nutrient medium. The two methodologies were compared by considering the direction of gene expression modulation for each transcript. As shown in Table S3 in the supplemental material, 13 of the 14 experimental points evaluated showed the same direction of gene expression modulation, indicating a 92.9% rate of coincidence between microarray and qRT-PCR data. In general, qRT-PCR experiments showed expression ratios higher than those observed in the microarrays, probably indicating a higher sensitivity of the former approach. The Spearman correlation obtained by considering all experimental data was 0.86, indicating a relatively strong correlation between microarray and qRT-PCR results.
Upregulation of genes during germination in inorganic salt solution relative to germination in nutrient medium.
Microarray hybridization analysis with RNA from cells isolated 30 min after induction of germination in the presence of adenine or potassium revealed 42 genes with higher transcript levels in both cases than in cells germinating in nutrient medium (Fig. 4). Among these genes, 26 were putatively identified and are listed in Table 4. Interestingly, most of these transcripts were already detected in the zoospores and their levels decreased during germination in nutrient medium, as observed in the time course germination assays.
TABLE 4.
Genes upregulated at 30 min after induction in cells germinating in the presence of adenine or potassium relative to their expression in cells germinating in nutrient medium
| Clone ID | Best alignment (Swiss-Prot); description | GO description | A ratioa | K ratiob |
|---|---|---|---|---|
| BeE90N01D10 | Q9Y823; probable homocitrate synthase, mitochondrial | Amino acid biosynthesis | 1.83 | 1.87 |
| BeE30N06E01 | Q34DS8; amino acid permease-associated region | Amino acid transport | 2.36 | 2.21 |
| BeE120N01A01 | Q3RIG0; amino acid permease-associated region | Amino acid transport | 1.10 | 1.13 |
| BeG30N13H09 | Q3WZJ7; amino acid permease-associated region | Amino acid transport | 1.97 | 1.90 |
| BeE30N10F07 | Q440D2; amino acid permease-associated region precursor | Amino acid transport | 1.76 | 1.83 |
| BeG60N01F09 | Q4WZ19; high affinity methionine permease | Amino acid transport | 1.30 | 0.97 |
| BeE120N09C08 | Q8P9N1; cationic amino acid transporter | Amino acid transport | 0.77 | 0.84 |
| BeE60H21G09 | Q8PLF9; cationic amino acid transporter | Amino acid transport | 2.01 | 2.21 |
| BeZSPN17H06 | O06479; YfnA | Amino acid transport | 2.96 | 2.84 |
| BeG30N15D05 | Q2SCN2; carbonic anhydrase | Carbon utilization | 0.694 | 1.81 |
| BeE30N14D03 | Q7ZUY3; histone H2A.x | Chromosome organization and biogenesis | 1.62 | 1.14 |
| BeE60H06B09 | Q4PEF9; H2A_NEUCR histone H2A | Chromosome organization and biogenesis | 1.83 | 1.25 |
| BeZSPN03D06 | Q9HDN1; histone H3 | Chromosome organization and biogenesis | 1.61 | 0.88 |
| BeE30N02C10 | Q4P7J7; H3_EMENI histone H3 | Chromosome organization and biogenesis | 2.13 | 1.31 |
| BeZSPN11B02 | Q2UFJ0; histone H4 | Chromosome organization and biogenesis | 2.01 | 1.24 |
| BeG90N18F10 | Q8SZ87; RE13747p | Mitosis | 1.04 | 1.02 |
| BeE60N03D06 | Q4WJ09; putative ketoreductase | Oxidation reduction | 1.75 | 0.95 |
| BeE60C17C02 | P38356; metal homeostatis protein BSD2 | Cation transport | 1.72 | 1.15 |
| BeZSPN09E08 | Q7Z8B6; H+-ATPase | Proton transport | 0.778 | 0.631 |
| BeZSPN10H02 | Q7RW00; hypothetical protein | Electron transport | 1.56 | 1.48 |
| BeE60N09D01 | Q2S1W1; acyl-coenzyme A oxidase I, putative | Oxidoreductase activity | 1.43 | 1.12 |
| BeZSPN14D10 | O93787; Chs3 | Transferase activity | 1.20 | 0.724 |
| BeZSPN02F03 | Q96338; AMP-binding protein | Fatty acid metabolism | 1.45 | 1.13 |
| BeE60N19B01 | Q95R88; SD01152p | Fatty acid metabolism | 2.00 | 1.01 |
| BeG3-0N06H02 | O74879; SPCC330.09 protein | rRNA processing | 1.41 | 1.42 |
| BeG30N09H07 | Q5KN22; ubiquitin-conjugating enzyme E2 | Ubiquitin cycle | 0.84 | 0.858 |
Log2 of the normalized expression ratios for cells germinating in adenine compared to cells germinating in nutrient medium.
Log2 of the normalized expression ratios for cells germinating in potassium compared to cells germinating in nutrient medium.
A total of eight genes that were upregulated in cells germinating in inorganic solution compared to their levels of expression in cells germinating in nutrient medium encode amino acid transporters (Table 4). The corresponding transcripts are present in the zoospores, with their levels decreasing during germination in nutrient medium but remaining high during germination triggered by adenine or potassium. These results support the idea that these amino acid transporters could play a role in nutrient sensing and that their expression would be negatively controlled by the presence of amino acids or other nutrients in the medium. Interestingly, Seong et al. (29) have described a similar finding during germination of the filamentous fungus Fusarium graminearum. These authors observed that more permeases and transporters are expressed in fresh spores and in hyphae, when nutrient-limiting conditions may exist, than in activated spores when the fungus is in a more nutrient-rich environment. Thus, the expression of amino acid transporter genes in both Blastocladiella and Fusarium is repressed in the presence of nutrients.
Five genes involved in chromosome organization and biogenesis show the same expression profile as the amino acid transporter genes (Table 4). This finding may be related to alterations in chromatin structure that are necessary for the unblocking of transcription and DNA replication that occurs during germination in nutrient medium and results in important alterations both in the control of the cell cycle and in the transcriptome of B. emersonii. Our results indicate that histones could be involved in the regulation of DNA transcription, acting selectively in the unblocking of the genes related to cell growth that are induced only in nutrient medium.
Genes implicated in fatty acid metabolism are also upregulated in cells germinating in inorganic solution compared to their levels of expression in cells germinating in nutrient medium, indicating that B. emersonii cells must use their endogenous lipid reserves in order to germinate in the absence of nutrients (Table 4). We also detected the induction of the gene encoding H+-ATPase, which generates the electrochemical gradient required for ion balance, nutrient uptake, and energy production in fungi; the gene for a carbonic anhydrase, related to carbon utilization and intracellular pH regulation; and the gene encoding a mitochondrial homocitrate synthase (Table 4). Homocitrate synthase is an enzyme that catalyzes the first reaction of the α-aminoadipate pathway, which is responsible for lysine biosynthesis in some fungi. Lysine may be converted to acetyl-coenzyme A, which then enters the tricarboxylic acid cycle. This observation suggests that when germination proceeds in the absence of nutrients, the fungus needs to synthesize metabolic precursors for energy production.
A larger number of genes are upregulated after 60 min of germination in the absence of nutrients, 68 of them being detected in the presence of both adenine and potassium (see Table S4 in the supplemental material). Among them we observed genes related to DNA catabolism, proteolysis, response to toxins, and apoptosis. Interestingly, 13 of these genes (see Table S4 in the supplemental material) are also upregulated in response to heat shock and/or cadmium stress, 6 of which are without a match in public databases (5). This finding indicates that at this time point, cells germinating in the absence of nutrients begin to express genes involved in stress responses.
Downregulated genes during germination in inorganic salt solution relative to germination in nutrient medium.
Several genes were downregulated in cells germinating in inorganic salt solution in comparison to their expression in cells germinating in the presence of nutrients. Consistently, these genes are mainly related to cell growth processes, such as glycolysis, protein biosynthesis, nucleotide biosynthesis, transcription, and transport (Table 5). As observed, these genes were induced during germination in the presence of energy-rich compounds in the induction medium (Fig. 2, K1 to K5), indicating that nutrients exert a positive regulatory effect on the transcription of these genes.
TABLE 5.
Functional GO categories observed among the genes downregulated when zoospores germinate in inorganic salt solution in comparison with their expression levels during germination in the presence of nutrients
| Gene function downregulated at indicated time point (min) of germination
| |
|---|---|
| 30 | 60 |
| Nucleosome assembly | Nucleosome assembly |
| Nucleotide biosynthesis | Regulation of transcription, DNA-dependent |
| Transcription | Transcription |
| rRNA processing | rRNA processing |
| Ribosome biogenesis | Ribosome biogenesis |
| Ribosome assembly | Translation initiation factor activity |
| Regulation of translational initiation | Protein biosynthesis |
| Translational termination | Protein targeting |
| Purine salvage | Protein-mitochondrial targeting |
| Arginine biosynthesis | Protein transport |
| Citrulline metabolism | Chaperone activity |
| Establishment of cell polarity | Transport |
| Cell motility | Pseudouridine synthesis |
| Cytokinesis | Small GTPase-mediated signal transduction |
| Glycolysis | |
| Ubiquitin-dependent protein catabolism | |
| Cell cycle | |
Final remarks.
Analyses of stage-specific gene expression and alterations in gene expression levels related to fungal life cycle stages are central to understanding the molecular mechanisms responsible for germination and other developmental processes in fungi. The data presented here provide important insights into the molecular mechanisms of fungal germination at the cellular level and contribute to a better understanding of regulation of gene expression related to this morphological transition in B. emersonii.
The question concerning the signal transduction pathways involved in triggering B. emersonii's germination process, for instance, was investigated by analyzing the expression pattern of the corresponding genes. The data revealed that a large number of distinct transcripts related to signal transduction are observed in zoospores and found to present the same expression profile during the initial stages of germination with all the inducers investigated. These results indicate that many signaling pathways are activated irrespective of the initial stimulus used to trigger this developmental stage.
The functional categories overrepresented among the genes upregulated during B. emersonii germination were found to coincide with those involved in the process of germination of other fungi. However, genes involved in cellular growth, including genes involved in protein biosynthesis and energetic metabolism, were only upregulated in germination induced in the presence of nutrients. In addition, many genes downregulated during germination in nutrient medium are kept at high expression levels during germination triggered in inorganic salt solution. These results indicate that nutrients exert an important role in the regulation of many genes during the germination process.
Furthermore, many transcripts encoding proteins without a match in public databases were found to be differentially expressed in our experiments and thus constitute an important initial functional characterization of the corresponding genes.
Supplementary Material
Acknowledgments
This work was supported by grants to S.L.G. from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). S.M.S.-I. was a doctoral fellow of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), T.K. was a doctoral fellow of FAPESP, and R.Z.N.V. was a doctoral fellow of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). S.L.G. was partially supported by CNPq.
S. M. Salem-Izacc is on leave from Instituto de Ciências Biológicas, Universidade Federal de Goiás, Goiás, Brazil.
Footnotes
Published ahead of print on 19 December 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Behrens, M. M., and J. C. C. Maia. 1986. Differentiation specific activation of cyclic AMP-dependent protein kinase in Blastocladiella emersonii. Biochem. Int. 12503-512. [Google Scholar]
- 2.Coutinho, E. C., and L. C. Correa. 1999. The induction of sporulation in the aquatic fungus Blastocladiella emersonii is dependent on extracellular calcium. FEMS Microbiol. Lett. 179353-359. [DOI] [PubMed] [Google Scholar]
- 3.d'Enfert, C. 1997. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21163-172. [Google Scholar]
- 4.Etchebehere, L. S., M. N. Simon, R. B. Campanhã, P. D. Zapella, M. Véron, and J. C. Maia. 1993. Developmental regulation of hexosamine biosynthesis by protein phosphatases 2A and 2C in Blastocladiella emersonii. J. Bacteriol. 1755022-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georg, R. C., and S. L. Gomes. 2007. Transcriptome analysis in response to heat shock and cadmium in the aquatic fungus Blastocladiella emersonii. Eukaryot. Cell 61053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes, S. L., L. Mennucii, and J. C. C. Maia. 1978. Adenylate cyclase and cyclic AMP metabolism during cytodifferentiation of Blastocladiella emersonii. Biochim. Biophys. Acta 541190-198. [DOI] [PubMed] [Google Scholar]
- 7.Gomes, S. L., L. Mennucci, and J. C. C. Maia. 1979. A calcium-dependent protein activator of mammalian cyclic nucleotide phosphodiesterase from Blastocladiella emersonii. FEBS Lett. 9939-42. [DOI] [PubMed] [Google Scholar]
- 8.Gomes, S. L., L. Mennucci, and J. C. C. Maia. 1980. Calcium efflux during germination of Blastocladiella emersonii. Dev. Biol. 77157-166. [DOI] [PubMed] [Google Scholar]
- 9.Gomes, S. L., L. Mennucci, and J. C. C. Maia. 1980. Induction of Blastocladiella emersonii germination by cyclic adenosine 3′,5′-monophosphate. Cell Differ. 9169-179. [Google Scholar]
- 10.Gong, C., and J. S. Lovett. 1977. Regulation of protein synthesis in Blastocladiella zoospores: factors for synthesis in nonsynthetic spores. Exp. Mycol. 1138-151. [Google Scholar]
- 11.Gottschalk, W. K., and D. R. Sonneborn. 1982. Phenotypic dissections of the Blastocladiella emersonii zoospore's developmental choice. Dev. Biol. 93165-180. [DOI] [PubMed] [Google Scholar]
- 12.Grunstein, M. 1990. Histone function in transcription. Annu. Rev. Cell Biol. 6643-676. [DOI] [PubMed] [Google Scholar]
- 13.Hein, C., and B. André. 1997. A C-terminal di-leucine motif and nearby sequences are required for NH4+-induced inactivation and degradation of the general amino acid permease, Gap1p, of Saccharomyces cerevisiae. Mol. Microbiol. 24607-616. [DOI] [PubMed] [Google Scholar]
- 14.Hollomon, D. W. 1970. Ribonucleic acid synthesis during fungal spore germination. J. Gen. Microbiol. 6275-87. [DOI] [PubMed] [Google Scholar]
- 15.Juliani, M. H., M. R. Brochetto, and J. C. Da Costa Maia. 1979. Changes in cyclic AMP binding and protein kinase activities during growth and differentiation of Blastocladiella emersonii. Cell Differ. 8421-430. [DOI] [PubMed] [Google Scholar]
- 16.Kasuga, T., J. P. Townsend, C. Tian, L. B. Gilbert, G. Mannhaupt, J. W. Taylor, and N. L. Glass. 2005. Long-oligomer microarray profiling in Neurospora crassa reveals the transcriptional program underlying biochemical and physiological events of conidial germination. Nucleic Acids Res. 336469-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinoshita, K., T. Nemoto, K. Nabeshima, H. Kondoh, H. Niwa, and M. Yanagida. 1996. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells 129-45. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita, N., H. Yamano, H. Niwa, T. Yoshida, and M. Yanagida. 1993. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 61059-1071. [DOI] [PubMed] [Google Scholar]
- 19.Koide, T., S. M. Salem-Izacc, S. L. Gomes, and R. Z. N. Vêncio. 2006. SpotWhatR: a user-friendly microarray data analysis system. Genet. Mol. Res. 593-107. [PubMed] [Google Scholar]
- 20.Leaver, C. J., and J. S. Lovett. 1974. An analysis of protein and RNA synthesis during encystment and outgrowth (germination) of Blastocladiella zoospores. Cell Differ. 3165-192. [DOI] [PubMed] [Google Scholar]
- 21.Liu, T., Q. Zhang, L. Wang, L. Yu, W. Leng, J. Yang, L. Chen, J. Peng, L. Ma, J. Dong, X. Xu, Y. Xue, Y. Zhu, W. Zhang, L. Yang, W. Li, L. Sun, Z. Wan, G. Ding, F. Yu, K. Tu, Z. Qian, R. Li, Y. Shen, Y. Li, and Q. Jin. 2007. The use of global transcriptional analysis to reveal the biological and cellular events involved in distinct development phases of Trichophyton rubrum conidial germination. BMC Genomics 8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 23.Lovett, J. S. 1968. Reactivation of ribonucleic acid and protein synthesis during germination of Blastocladiella zoospores and the role of the ribosomal nuclear cap. J. Bacteriol. 96962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maia, J. C. C., and E. P. Camargo. 1974. cAMP phosphodiesterase activity during growth and differentiation in Blastocladiella emersonii. Cell Differ. 3147-155. [DOI] [PubMed] [Google Scholar]
- 25.Marques, M. V., A. C. C. Borges, J. C. F. Oliveira, and S. L. Gomes. 1992. Coordinate pretranslational control of cAMP-dependent protein kinase subunit expression during development in the water mold Blastocladiella emersonii. Dev. Biol. 149432-439. [DOI] [PubMed] [Google Scholar]
- 26.Mayer-Jaekel, R. E., and B. A. Hemmings. 1994. Protein phosphatase 2A: a “ménage à trois”. Trends Cell Biol. 4287-291. [DOI] [PubMed] [Google Scholar]
- 27.Osherov, N., and G. S. May. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199153-160. [DOI] [PubMed] [Google Scholar]
- 28.Ribichich, K. F., S. M. Salem-Izacc, R. C. Georg, R. Z. N. Vêncio, L. D. Navarro, and S. L. Gomes. 2005. Gene discovery and expression profile analysis through sequencing of expressed sequence tags from different developmental stages of the chytridiomycete Blastocladiella emersonii. Eukaryot. Cell 4455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seong, K. Y., X. Zhao, J. R. Xu, U. Güldener, and H. C. Kistler. 2008. Conidinal germination in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 45389-399. [DOI] [PubMed] [Google Scholar]
- 30.Silva, A. M., J. C. Maia, and M. H. Juliani. 1987. Changes in the pattern of protein synthesis during zoospore germination in Blastocladiella emersonii. J. Bacteriol. 1692069-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman, P. M., and P. M. Epstein. 1975. Cyclic nucleotide metabolism coupled to cytodifferentiation of Blastocladiella emersonii. Proc. Natl. Acad. Sci. USA 72442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman, P. M., M. M. Huh, and L. Sun. 1974. Protein synthesis during zoospore germination in the aquatic phycomycete Blastocladiella emersonii. Dev. Biol. 4059-70. [DOI] [PubMed] [Google Scholar]
- 33.Simão, R. C., and S. L. Gomes. 2001. Structure, expression, and functional analysis of the gene coding for calmodulin in the chytridiomycete Blastocladiella emersonii. J. Bacteriol. 1832280-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, J. D., and P. M. Silverman. 1973. Lipid turnover during morphogenesis in the water mold Blastocladiella emersonii. Biochem. Biophys. Res. Commun. 541191-1197. [DOI] [PubMed] [Google Scholar]
- 35.Soll, D. R., and D. R. Sonneborn. 1969. Zoospore germination in the water mold Blastocladiella emersonii. II. Influence of cellular and environmental variables on germination. Dev. Biol. 20218-235. [DOI] [PubMed] [Google Scholar]
- 36.Soll, D. R., R. Bromberg, and D. R. Sonneborn. 1969. Zoospore germination in the water mold Blastocladiella emersonii. I. Measurement of germination and sequence of subcellular morphological changes. Dev. Biol. 20183-217. [DOI] [PubMed] [Google Scholar]
- 37.Soll, D. R., and D. R. Sonneborn. 1971. Zoospore germination in Blastocladiella emersonii: cell differentiation without protein synthesis? Proc. Natl. Acad. Sci. USA 68459-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soll, D. R., and D. R. Sonneborn. 1971. Zoospore germination in Blastocladiella emersonii. III. Structural changes in relation to protein and RNA synthesis. J. Cell Sci. 9679-699. [DOI] [PubMed] [Google Scholar]
- 39.Stanbrough, M., and B. Magasanik. 1995. Role of the GATA factors Gln3p and Nil1p of Saccharomyces cerevisiae in the expression of nitrogen-regulated genes. Proc. Natl. Acad. Sci. USA 929450-9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanbrough, M., and B. Magasanik. 1995. Transcriptional and posttranslational regulation of the general amino acid permease of Saccharomyces cerevisiae. J. Bacteriol. 17794-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truesdell, L. C., and E. C. Cantino. 1971. The induction and early events of germination in the zoospore of Blastocladiella emersonii. Curr. Top. Dev. Biol. 61-44. [DOI] [PubMed] [Google Scholar]
- 42.Vale, M. R., and J. C. Maia. 1976. Changes in cGMP phosphodiesterase levels during growth and differentiation in Blastocladiella emersonii. FEBS Lett. 70205-208. [DOI] [PubMed] [Google Scholar]
- 43.Vale, V. L., S. L. Gomes, J. C. C. Maia, and L. Mennuci. 1976. Transient cyclic AMP accumulation in germinating zoospores of Blastocladiella emersonii. FEBS Lett. 67189-192. [DOI] [PubMed] [Google Scholar]
- 44.Van Brunt, J., and F. M. Harold. 1980. Ionic control of germination of Blastocladiella emersonii zoospores. J. Bacteriol. 141735-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandercammen, A., J. M. François, B. B. Torres, J. C. Maia, and H. G. Hers. 1990. Fructose 2,6-biphosphate and carbohydrate metabolism during the life cycle of the aquatic fungus Blastocladiella emersonii. J. Gen. Microbiol. 136137-146. [DOI] [PubMed] [Google Scholar]
- 46.Vêncio, R. Z. N., and T. Koide. 2005. HTself: self-self based statistical test for low replication microarray studies. DNA Res. 12211-214. [DOI] [PubMed] [Google Scholar]
- 47.Vêncio, R. Z. N., T. Koide, S. L. Gomes, and C. A. B. Pereira. 2006. BayGO: Bayesian analysis of ontology term enrichment in microarray data. BMC Bioinformatics 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendland, J. 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 3463-82. [DOI] [PubMed] [Google Scholar]
- 49.Williams, R. J. 1992. Calcium and calmodulin. Cell Calcium 13355-362. [DOI] [PubMed] [Google Scholar]
- 50.Zahiri, A. R., M. R. Babu, and B. J. Saville. 2005. Differential gene expression during teliospore germination in Ustilago maydis. Mol. Genet. Genomics 273394-403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.