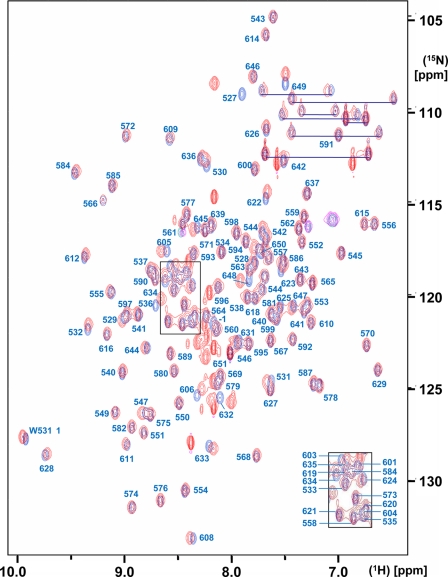
FIG. 1.
Superposition of the 2D [15N,1H]-HSQC spectra of SUD-M(513-651) (red) and SUD-M(527-651) (blue). The protein concentrations were 1.2 mM and 1.4 mM for SUD-M(513-651) and SUD-M(527-651), respectively. The solvent contained 25 mM sodium phosphate buffer at pH 6.5, 150 mM NaCl, and 2 mM NaN3. The spectra were recorded at a 1H frequency of 600 MHz and a temperature of 25°C, with 256 increments in the 15N dimension and 4 scans/increment. The resonance assignments for SUD-M(527-651) are marked in blue, where the assignments for the crowded central region are shown as an insert in the lower right corner. Residue −1 indicates the methionine residue of the tetrapeptide segment −4GSHM−1 that is left after thrombin cleavage (see the text). The side-chain amide resonances of asparagine and glutamine are connected by blue horizontal lines.