Abstract
Increased transgene expression per vector genome is an important goal in the optimization of viral vectors for gene therapy. Herein we demonstrate that herpes simplex virus type 1 (HSV1) thymidine kinase (TK) gene sequences (1,131 bp) fused to the 3′ end of lacZ increase transgene expression from high-capacity adenoviral vectors (HCAd), but not from first-generation (Ad) vectors. The woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), in contrast, increased transgene expression levels from Ad but not HCAd vectors. The differential activity of the HSV1 TK gene and WPRE sequences was detected both in vitro and in vivo and suggests potentially different mechanisms of action or the interaction of these elements with vector genomic sequences.
Adenoviral vectors are effective vectors for gene transfer and gene therapy. However, adenoviral vectors induce dose-dependent innate and adaptive immune responses (24, 33, 34, 41, 46, 50, 51). Decreasing the total dose of therapeutically effective viral vector should result in safer and longer-term gene transfer. To do so, it is desirable to increase the levels of transgene expression per vector genome (vg). This can be achieved through the use of genetic sequences that either increase the number of transcript copies (e.g., stronger promoters) or stabilize the mRNA (e.g., the woodchuck hepatitis virus posttranscriptional regulatory element [WPRE]) (3, 16, 21, 22, 26, 29, 30, 32, 40, 48, 52).
Previously, we reported that intracranial delivery of a first-generation adenoviral vector encoding herpes simplex virus type 1 (HSV1) thymidine kinase (Ad-TK) resulted in unexpectedly high levels of gene expression, widespread distribution, and strong biological activity, suggesting that HSV1 TK has the capacity to upregulate transgene expression (11). Moreover, in the same report we demonstrate that an adenoviral vector with a 60-bp deletion in the coding sequence for the 5′ region of TK elicits decreased levels of transgene expression and biological activity compared to the vector encoding wild-type HSV1 TK (8, 11).
To determine if HSV1 TK gene sequences could upregulate transgene expression when provided in trans, we coinjected Ad-TK mixed with an Ad vector expressing the β-galactosidase (β-Gal) reporter gene (49). Our results suggested that the HSV1 TK gene sequence provided in trans failed to increase transgene expression from the vectors expressing β-Gal. So far, the use of cis-acting HSV1 TK gene elements in viral vectors to increase transgene expression has not been investigated, although elements within the HSV1 TK gene have been shown to act as pre-mRNA processing enhancers to increase expression of intronless genes when tested in plasmids.
In the present work we investigated whether HSV1 TK gene sequences enhance transgene expression from either first-generation adenoviral vectors (Ad) or high-capacity, helper-dependent adenoviral vectors (HCAd) and compared the activity of these gene sequences to the activity of an established posttranscriptional regulatory sequence (i.e., WPRE). To do so, we engineered expression cassettes carrying the reporter transgene (lacZ) under the control of the powerful murine cytomegalovirus (mCMV) promoter with potential regulatory sequences linked to the 3′ end of lacZ. The regulatory sequences tested were as follows: HSV1 TK gene (1,131 bp); the ΔTK gene, a truncated form of the HSV1 TK gene (1,072 bp) with a 60-bp deletion downstream of the first initiation codon; and WPRE (594 bp), used as a control posttranscriptional regulatory element (3, 16, 30, 39, 48). A control construct of lacZ without a posttranscriptional sequence was also engineered.
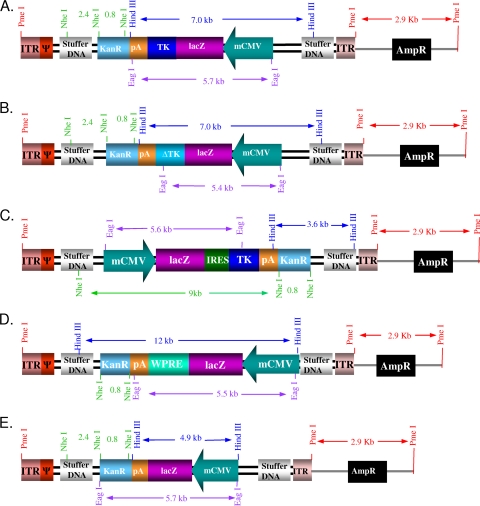
The expression cassettes were utilized to generate the first-generation Ad vectors Ad-mCMV.βgal.TK, Ad-mCMV.βgal.ΔTK, Ad-mCMV.βgal.WPRE, and Ad-mCMV.βgal, respectively, using methodologies described by us previously (43). The same expression cassettes (Fig. 1) were also used to generate the high-capacity, helper-dependent adenoviral vectors HCAd-mCMV.βgal.TK, HCAd-mCMV.βgal.ΔTK, HCAd-mCMV.βgal.WPRE, and HCAd-mCMV.βgal using methodologies described by us previously (35, 37). We also generated a fifth HCAd vector, HCAd-mCMV.βgal.IRES.TK, designed to express both β-Gal and TK. Briefly, four of the vectors are completely new and have never been published before: HCAd-mCMV.βgal.ΔTK, HCAd-mCMV.βgal.IRES.TK, Ad-mCMV.βgal.TK, and Ad-mCMV.βgal.ΔTK. Three other vectors, HCAd-mCMV.βgal, HCAd-mCMV.βgal.TK, and HCAd-mCMV.βgal.WPRE, have been used before, but only to assess genome levels in the development of a novel method for the quantitation and titration of Ad vectors (39), even though their biological activity was neither evaluated nor reported previously. The expression levels of only two vectors (Ad-mCMV.βgal and Ad-mCMV.βgal.WPRE) were described by our group in detail previously (3, 15), and these vectors are included here only as controls. Thus, in this paper we report four completely novel vectors never described before and, for the first time, the biological activity for seven vectors. A total of nine vectors were used in this work.
FIG. 1.
Linear depiction of the HCAd vectors carrying the various mCMV-driven lacZ cassettes containing potential regulatory sequences. The constructs indicate the individual components and the orientations of the cassettes and their promoters. Relevant restriction sites are shown. (A) HCAd-mCMV.βgal.TK; (B) HCAd-mCMV.βgal.ΔTK; (C) HCAd-mCMV.βgal.IRES.TK; (D) HCAd-mCMV.βgal.WPRE; (E) HCAd-mCMV.βgal. Although the cassettes within HCAd are shown, the identical cassettes were utilized in first-generation Ad. For space considerations, these are not illustrated. See the supplemental material for details of vector construction. ITR, inverted terminal repeat; IRES, internal ribosome entry site.
Integrity of the cassettes was confirmed by sequencing (data not shown). All vectors were titered in parallel for blue forming units (BFU) and vgs and were certified free of contaminating lipopolysaccharide and replication-competent adenovirus as described before (12, 39, 43). The vectors obtained were titrated, and the values obtained are shown in Table 1.
TABLE 1.
Titer of viral preparations by BFU and vgs
| Vector | BFU/ml | vgs/ml |
|---|---|---|
| Ad-mCMV.βgal | 1.64 × 1011 | 2.21 × 1012 |
| Ad-mCMV.βgal.TK | 3.28 × 1011 | 1.60 × 1012 |
| Ad-mCMV.βgal.ΔTK | 1.32 × 1012 | 2.70 × 1013 |
| Ad-mCMV.βgal.WPRE | 5.12 × 109 | 4.97 × 1010 |
| HCAd-mCMV.βgal | 4.27 × 109 | 3.68 × 1011 |
| HCAd-mCMV.βgal.TK | 7.66 × 109 | 6.96 × 1010 |
| HCAd-mCMV.βgal.ΔTK | 1.01 × 1011 | 4.37 × 1012 |
| HCAd-mCMV.βgal.IRES.TK | 2.46 × 1011 | 1.29 × 1013 |
| HCAd-mCMV.βgal.WPRE | 2.44 × 1011 | 3.63 × 1012 |
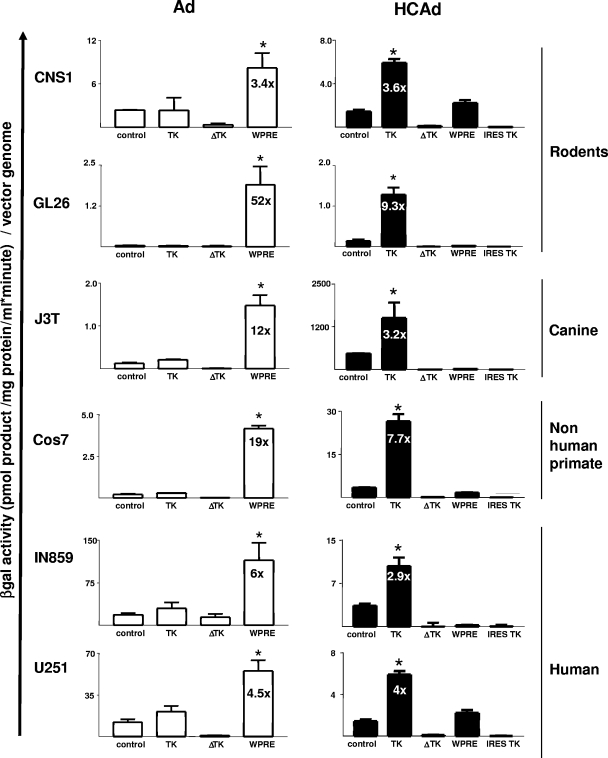
To determine the optimal, nonsaturating vector dose, we generated a dose-response curve using the cell lines CNS1 (Lewis rat glioma), GL26 (C57BL/6 mouse glioma), J3T (canine glioma), Cos7 (primate kidney, as a control), IN859 (human glioma), and U251 (human glioma) (data not shown) at multiplicities of infection (MOIs) ranging from 30 to 1,000 genomes per cell. Based on the linear range of expression for all vectors, an MOI of 300 vgs/cell was chosen for further detailed analysis. Expression of β-Gal and β-Gal activity were tested in all cell lines as described elsewhere (1, 3, 7, 47). For all subsequent data analysis, results were standardized and reported as β-Gal activity per vg. All data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test.
When carried within a first-generation Ad vector, the HSV1 TK gene sequences did not increase β-Gal activity per vg in any of the cell lines used for the study (Fig. 2, left). The enhancement provided by WPRE was similar to results reported by others (25, 36).
FIG. 2.
In vitro transgene expression is upregulated by the HSV1 TK gene sequence carried in HCAd (right) but not in Ad (left), while WPRE increased expression in Ad (left), but not in HCAd (right). Rat (CNS-1), mouse (GL26), and dog (J3T) glioma cells, monkey kidney cells (Cos7), and human glioma cells (IN859 and U251) were infected at an MOI of 300 vgs/cell. Cells were incubated for 72 h, and transgene expression was determined by β-Gal activity assay of cell lysates. Experiments were performed in triplicate. Numbers of vgs were determined for all the viral preparations using quantitative PCR. Bars represent the means ± standard errors of the means of β-Gal activity, calculated as o-nitrophenol produced (pmol)/(sample protein content [mg/ml] × incubation time (min), per inoculated vg. Data were analyzed by one-way ANOVA followed by the Tukey-Kramer multiple comparison test. *, P < 0.05 versus control group (cells infected with the corresponding vector bearing the mCMV-β-Gal cassette).
However, when contained within HCAd vectors, the HSV1 TK gene sequence provided increases in β-Gal activity per genome that ranged from 2.9- to 9.3-fold. However, no increased β-Gal activity per vg was detected using the ΔTK gene sequence in HCAds. The inability of the ΔTK gene sequence to enhance transgene expression from the HCAd vector backbones could indicate that the 60 bp at the 5′ extreme of the HSV1 TK gene is required for the enhanced expression provided. We also compared the β-Gal activity per vg mediated by infection with HCAd-mCMV.βgal.IRES.TK to determine if the HSV1 TK gene sequence separated from the β-Gal transgene by an internal ribosome entry site or the HSV1 TK protein potentially expressed from an internal ribosome entry site could increase levels of transgene expression. With this vector, no increased β-Gal activity was observed.
Unlike increased transgene expression provided by WPRE when carried by first-generation Ad vectors, there was no effect of WPRE on transgene expression when it was carried by the HCAd vector (Fig. 2, right). The same shuttle vector was used to generate both Ad and HCAd vectors, and sequence analysis was used to confirm that the sequences of the WPREs in both cassettes were identical (data not shown). Although WPRE has recently been used by several groups in HCAd vectors in conjunction with tissue-specific promoters (6, 18, 20), a direct comparison of transgene expression levels from HCAds with and without the WPRE has not been performed. Our data indicate that, under our experimental conditions, and compared side by side, WPRE failed to increase transgene expression from HCAd.
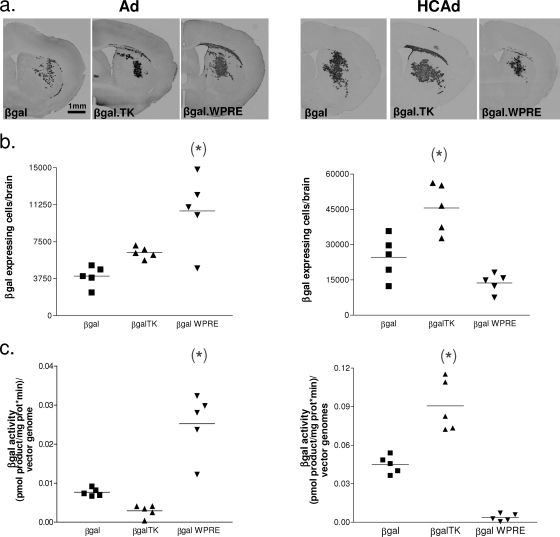
We further tested the effects of the HSV1 TK sequence on transgene expression from HCAd and Ad vectors in vivo. Adult female C57BL/6 mice with body weights between 18 and 25 g were used. Five mice per group (n = 5) were injected with 1.0 × 106 vgs of vectors that had shown increased transgene expression due to either the HSV1 TK gene or WPRE and their respective control vectors (i.e., HCAd-mCMV.βgal, HCAd-mCMV.βgal.TK, and HCAd-mCMV.βgal.WPRE and Ad-mCMV.βgal, Ad-mCMV.βgal.TK, and Ad-mCMV.βgal.WPRE) into the striatum as described previously (43, 47). For immunohistochemistry, 5 days postinjection, animals were perfused with 100 ml oxygenated Tyrode's solution followed by a 4% paraformaldehyde solution. Brains were serially sectioned using an electronic Vibratome (Leica) to obtain 50-μm free-floating sections. Serial sections were immunoreacted using a rabbit polyclonal anti-β-Gal primary antibody (diluted 1:1,000; generated in our laboratory [2, 42-44]). A series of sections spanning the entire injection site (and containing β-Gal-expressing cells), separated from each other by 250 μm, were quantified by unbiased quantitative stereology using Microbrightfield software on a Zeiss upright microsocope.
We obtained a 2.64-fold increase of β-Gal-expressing cells per vg in the brains of mice injected with Ad-mCMV.βgal.WPRE into the striatum, compared to the level obtained with the control vector Ad-mCMV.βgal (Fig. 3a and b, left). As shown in the in vitro experiments, the HSV1 TK gene sequence within Ad failed to increase transgene expression per vg in vivo. We found a 1.8-fold increase in the number of β-Gal-expressing cells per vg in the brains of mice stereotactically injected with HCAd-mCMV.βgal.TK into the striatum, compared to the number in brains injected with the control vector HCAd-mCMV.βgal (Fig. 3a and b, right); as in the in vitro experiments, the WPRE within HCAd failed to increase transgene expression per vg in vivo.
FIG. 3.
In vivo transgene expression is upregulated by the HSV1 TK gene sequence carried in HCAd vectors, but not in Ad vectors. Five mice per group were stereotactically injected with 1.0 × 106 vgs of each of the high-capacity adenoviral vectors HCAd-mCMV.βgal, HCAd-mCMV.βgal.TK, and HCAd-mCMV.βgal.WPRE and with Ad-mCMV.βgal, Ad-mCMV.βgal.TK, and Ad-mCMV.βgal.WPRE. (a) Low-power microphotographs showing β-Gal immunoreactivity in representative striatal sections from mice injected intracranially with the different vectors; (b) Numbers of β-Gal-expressing cells per brain. The β-Gal staining was performed 5 days after injection, when animals were perfusion fixed, and transgene expression was determined by immunocytochemistry. The data are expressed as β-Gal-expressing cells per mouse striatum. β-Gal-expressing cells were quantified utilizing Stereo Investigator software, version 5.00 (Microbrightfield, Inc., Colchester, VT). (c) Five mice per group were stereotactically injected with 1.0 × 106 vgs of each of the high-capacity adenoviral vectors HCAd-mCMV.βgal, HCAd-mCMV.βgal.TK, and HCAd-mCMV.βgal.WPRE and with Ad-mCMV.βgal, Ad-mCMV.βgal.TK, and Ad-mCMV.βgal.WPRE. The enzymatic activity (β-Gal activity in brain lysates) was measured 3 days after intracranial injection of vectors, following animal perfusion with oxygenated Tyrode's solution. After testing for normality, all data were analyzed by one-way ANOVA followed by the Tukey-Kramer multiple comparison test. *, P < 0.05 versus control group, injected with a β-Gal-encoding vector.
This experiment was repeated, but injected brains were homogenized and β-Gal enzymatic activity was measured (Fig. 3c). Results from these experiments were comparable to those in which the transgene-expressing cells were counted, i.e., the WPRE increased β-Gal activity per genome from Ad and the HSV1 TK gene increased β-Gal activity per genome from HCAd (Fig. 3c). Data were analyzed by one-way ANOVA followed by the Tukey-Kramer multiple comparison test.
In previous publications authors have characterized elements found within the HSV1 TK gene that could act as posttranscriptional enhancers to increase expression from intronless genes. In detail, Liu and Mertz described a 119-bp element from the HSV1 TK gene that can act in cis to provide expression to intronless genes when carried upstream of a desired gene; this was done using in vitro transfections of plasmids (25). Guang et al. further demonstrated that a sequence, named the pre-mRNA processing enhancer, derived from the HSV1 TK gene enhances both polyadenylation and nucleocytoplasmic export of intronless mRNAs; this work also utilized only in vitro assays of plasmid DNA transfections (17). Finally, Otero and Hope have shown that sequences from the HSV1 TK gene behave similarly to WPRE by enhancing levels of expression of another construct; this work was also restricted to in vitro plasmid DNA transfections (36). Intriguingly, although these papers strived to isolate minimal elements conferring increased gene expression, they also showed that a longer HSV1 TK gene provided the same benefits; this longer HSV1 TK gene sequence, moreover, seems to be devoid of the initial 60 bp, which we determined to be required to provide increased expression from HCAd.
Taken together, our in vitro and in vivo results demonstrate that the HSV1 TK gene sequence increases levels of transgene expression per vg from high-capacity but not first-generation adenoviral vectors. Our results also suggest that the 60 bp at the 5′ extreme of the HSV1 TK gene may have an important role in the enhancement of transgene expression by our full-length HSV1 TK gene, since their deletion abolishes the enhancement described. Additionally, our data indicate that HSV1-derived sequences that regulate transgene expression from HCAd may differ from those previously shown to increase expression from intronless genes in plasmids (25, 36).
Immune responses to viral vectors constitute one of the major limitations of gene therapy. Recent improvements in vectors to overcome immunological challenges, such as the development of strong promoters, PEGylation of viral capsids, and the HCAd vector system, have improved the safety profile of viral vectors (4, 9, 10, 14, 31). Additional increases in transgene expression per vg would allow further reduction of the viral vector doses needed to achieve a therapeutic efficacy. WPRE has been systematically evaluated as a posttranscriptional regulatory element to increase transgene expression in adenoviral (3, 5, 30, 48), retroviral (19, 23), lentiviral (13, 29, 32, 40), and adeno-associated virus vectors (26, 27, 38, 45). Although WPRE has been used in HCAd vectors, transgene expression was not compared with that by the non-WPRE-containing vector version (6, 18, 20). In these various vector systems, the WPRE has been shown to increase transgene expression when tested with constitutive or cell-type-specific promoters. In these models and vectors, WPRE increased expression by 3- to 10-fold, similar to the increased expression from first-generation Ad vectors observed in our experiments. However, when carried in the HCAd vector backbone, WPRE failed to enhance transgene expression. One potential explanation could be that adenoviral sequences and/or gene products, which are absent from HCAd vectors, are necessary for WPRE to upregulate transgene expression (28). Whether differential interactions between adenoviral vector sequences and/or gene products and elements such as WPRE or the HSV1 TK gene are necessary for increased transgene expression remains to be determined.
In summary, while our data do not directly prove that the HSV1 TK gene sequence acts as a posttranscriptional regulatory element, the enhancement of HCAd-mediated transgene expression in the presence of the HSV1 TK gene sequence both in vitro and in vivo suggests that the HSV1 TK gene sequence could act as a posttranscriptional regulatory sequence when used in conjunction with HCAd vector systems. These data demonstrate the utility of a novel HSV-1-derived sequence that can act to increase transgene expression in the background of high-capacity adenoviral vectors.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) grant 1R01 NS44556.01, minority supplement NS445561, 1R21-NSO54143.01, and 1UO1 NS052465.01 to M.G.C. and by NIH/NINDS grants 1 RO1 NS 054193.01, RO1 NS 42893.01, and 1R21 NS047298-01 to P.R.L. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics provided support to P.R.L. and M.G.C., respectively. Support was also received from the Linda Tallen and David Paul Kane Foundation Annual Fellowship and the Board of Governors at CSMC. P.N. is supported by R01DK067324.
We thank S. Melmed, L. Fine, and R. Katzman for their support and academic leadership. We thank Marianela Candolfi for consultation regarding statistical analysis of our data. We acknowledge the critical contribution to this project by John Young and the Comparative Medicine staff at the CSMC.
We have no conflicting financial interests.
Footnotes
Published ahead of print on 10 December 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ali, S., J. F. Curtin, J. M. Zirger, W. Xiong, G. D. King, C. Barcia, C. Liu, M. Puntel, S. Goverdhana, P. R. Lowenstein, and M. G. Castro. 2004. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol. Ther. 101071-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, S., G. D. King, J. F. Curtin, M. Candolfi, W. Xiong, C. Liu, M. Puntel, Q. Cheng, J. Prieto, A. Ribas, J. Kupiec-Weglinski, N. van Rooijen, H. Lassmann, P. R. Lowenstein, and M. G. Castro. 2005. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 657194-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleby, C. E., P. A. Kingston, A. David, C. A. Gerdes, P. Umana, M. G. Castro, P. R. Lowenstein, and A. M. Heagerty. 2003. A novel combination of promoter and enhancers increases transgene expression in vascular smooth muscle cells in vitro and coronary arteries in vivo after adenovirus-mediated gene transfer. Gene Ther. 101616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcia, C., M. Jimenez-Dalmaroni, K. M. Kroeger, M. Puntel, A. J. Rapaport, D. Larocque, G. D. King, S. A. Johnson, C. Liu, W. Xiong, M. Candolfi, S. Mondkar, P. Ng, D. Palmer, M. G. Castro, and P. R. Lowenstein. 2007. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol. Ther. 152154-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulos, S., B. P. Meloni, P. G. Arthur, C. Bojarski, and N. W. Knuckey. 2006. Assessment of CMV, RSV and SYN1 promoters and the woodchuck post-transcriptional regulatory element in adenovirus vectors for transgene expression in cortical neuronal cultures. Brain Res. 110227-38. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti-Pierri, N., D. J. Palmer, V. Mane, M. Finegold, A. L. Beaudet, and P. Ng. 2005. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol. Ther. 1299-106. [DOI] [PubMed] [Google Scholar]
- 7.Candolfi, M., J. F. Curtin, W. D. Xiong, K. M. Kroeger, C. Liu, A. Rentsendorj, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 14371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowsill, C., T. D. Southgate, G. Morrissey, R. A. Dewey, A. E. Morelli, T. C. Maleniak, Z. Forrest, D. Klatzmann, G. W. Wilkinson, P. R. Lowenstein, and M. G. Castro. 2000. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Ther. 7679-685. [DOI] [PubMed] [Google Scholar]
- 9.Croyle, M. A., N. Chirmule, Y. Zhang, and J. M. Wilson. 2002. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 131887-1900. [DOI] [PubMed] [Google Scholar]
- 10.Croyle, M. A., H. T. Le, K. D. Linse, V. Cerullo, G. Toietta, A. Beaudet, and L. Pastore. 2005. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 12579-587. [DOI] [PubMed] [Google Scholar]
- 11.Dewey, R. A., G. Morrissey, C. M. Cowsill, D. Stone, F. Bolognani, N. J. Dodd, T. D. Southgate, D. Klatzmann, H. Lassmann, M. G. Castro, and P. R. Lowenstein. 1999. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 51256-1263. [DOI] [PubMed] [Google Scholar]
- 12.Dion, L. D., J. Fang, and R. I. Garver, Jr. 1996. Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J. Virol. Methods 56:99-107. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy, F. P., E. Mouly, M. Mesel-Lemoine, C. Morel, J. Abriol, M. Cherai, C. Baillou, D. Negre, F. L. Cosset, D. Klatzmann, and F. M. Lemoine. 2005. Lentiviral transduction of human hematopoietic cells by HIV-1- and SIV-based vectors containing a bicistronic cassette driven by various internal promoters. J. Gene Med. 71158-1171. [DOI] [PubMed] [Google Scholar]
- 14.Gahery-Segard, H., V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J. G. Guillet, and F. Farace. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 1002218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerdes, C. A., M. G. Castro, and P. R. Lowenstein. 2000. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol. Ther. 2330-338. [DOI] [PubMed] [Google Scholar]
- 16.Glover, C. P., A. S. Bienemann, D. J. Heywood, A. S. Cosgrave, and J. B. Uney. 2002. Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol. Ther. 5509-516. [DOI] [PubMed] [Google Scholar]
- 17.Guang, S., A. M. Felthauser, and J. E. Mertz. 2005. Binding of hnRNP L to the pre-mRNA processing enhancer of the herpes simplex virus thymidine kinase gene enhances both polyadenylation and nucleocytoplasmic export of intronless mRNAs. Mol. Cell. Biol. 256303-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermening, S., S. Kugler, M. Bahr, and S. Isenmann. 2006. Improved high-capacity adenoviral vectors for high-level neuron-restricted gene transfer to the CNS. J. Virol. Methods 13630-37. [DOI] [PubMed] [Google Scholar]
- 19.Hlavaty, J., M. Schittmayer, A. Stracke, G. Jandl, E. Knapp, B. K. Felber, B. Salmons, W. H. Gunzburg, and M. Renner. 2005. Effect of posttranscriptional regulatory elements on transgene expression and virus production in the context of retrovirus vectors. Virology 3411-11. [DOI] [PubMed] [Google Scholar]
- 20.Huang, B., J. Schiefer, C. Sass, G. B. Landwehrmeyer, C. M. Kosinski, and S. Kochanek. 2007. High-capacity adenoviral vector-mediated reduction of huntingtin aggregate load in vitro and in vivo. Hum. Gene Ther. 18303-311. [DOI] [PubMed] [Google Scholar]
- 21.Johansen, J., J. Tornoe, A. Moller, and T. E. Johansen. 2003. Increased in vitro and in vivo transgene expression levels mediated through cis-acting elements. J. Gene Med. 51080-1089. [DOI] [PubMed] [Google Scholar]
- 22.Ketteler, R., S. Glaser, O. Sandra, U. M. Martens, and U. Klingmuller. 2002. Enhanced transgene expression in primitive hematopoietic progenitor cells and embryonic stem cells efficiently transduced by optimized retroviral hybrid vectors. Gene Ther. 9477-487. [DOI] [PubMed] [Google Scholar]
- 23.Klein, R., B. Ruttkowski, E. Knapp, B. Salmons, W. H. Gunzburg, and C. Hohenadl. 2006. WPRE-mediated enhancement of gene expression is promoter and cell line specific. Gene 372153-161. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Q., A. K. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. J. Wickham, and D. A. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14627-643. [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., and J. E. Mertz. 1995. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes Dev. 91766-1780. [DOI] [PubMed] [Google Scholar]
- 26.Loeb, J. E., W. S. Cordier, M. E. Harris, M. D. Weitzman, and T. J. Hope. 1999. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum. Gene Ther. 102295-2305. [DOI] [PubMed] [Google Scholar]
- 27.Martin, K. R., H. A. Quigley, D. J. Zack, H. Levkovitch-Verbin, J. Kielczewski, D. Valenta, L. Baumrind, M. E. Pease, R. L. Klein, and W. W. Hauswirth. 2003. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 444357-4365. [DOI] [PubMed] [Google Scholar]
- 28.Martina, Y., D. Avitabile, S. Piersanti, G. Cherubini, and I. Saggio. 2007. Different modulation of cellular transcription by adenovirus 5, DeltaE1/E3 adenovirus and helper-dependent vectors. Virus Res. 13071-84. [DOI] [PubMed] [Google Scholar]
- 29.Mautino, M. R., and R. A. Morgan. 2002. Enhanced inhibition of human immunodeficiency virus type 1 replication by novel lentiviral vectors expressing human immunodeficiency virus type 1 envelope antisense RNA. Hum. Gene Ther. 131027-1037. [DOI] [PubMed] [Google Scholar]
- 30.Mian, A., W. M. McCormack, Jr., V. Mane, S. Kleppe, P. Ng, M. Finegold, W. E. O'Brien, J. R. Rodgers, A. L. Beaudet, and B. Lee. 2004. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol. Ther. 10492-499. [DOI] [PubMed] [Google Scholar]
- 31.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J. G. Guillet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 747678-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau-Gaudry, F., P. Xia, G. Jiang, N. P. Perelman, G. Bauer, J. Ellis, K. H. Surinya, F. Mavilio, C. K. Shen, and P. Malik. 2001. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood 982664-2672. [DOI] [PubMed] [Google Scholar]
- 33.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 151157-1166. [DOI] [PubMed] [Google Scholar]
- 34.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10965-976. [DOI] [PubMed] [Google Scholar]
- 35.Ng, P., R. J. Parks, and F. L. Graham. 2002. Preparation of helper-dependent adenoviral vectors. Methods Mol. Med. 69371-388. [DOI] [PubMed] [Google Scholar]
- 36.Otero, G. C., and T. J. Hope. 1998. Splicing-independent expression of the herpes simplex virus type 1 thymidine kinase gene is mediated by three cis-acting RNA subelements. J. Virol. 729889-9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer, D., and P. Ng. 2003. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 8846-852. [DOI] [PubMed] [Google Scholar]
- 38.Peden, C. S., C. Burger, N. Muzyczka, and R. J. Mandel. 2004. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 786344-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puntel, M., J. F. Curtin, J. M. Zirger, A. K. Muhammad, W. Xiong, C. Liu, J. Hu, K. M. Kroeger, P. Czer, S. Sciascia, S. Mondkar, P. R. Lowenstein, and M. G. Castro. 2006. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum. Gene Ther. 17531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramezani, A., T. S. Hawley, and R. G. Hawley. 2000. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol. Ther. 2458-469. [DOI] [PubMed] [Google Scholar]
- 41.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3708-722. [DOI] [PubMed] [Google Scholar]
- 42.Smith-Arica, J. R., J. C. Williams, D. Stone, J. Smith, P. R. Lowenstein, and M. G. Castro. 2001. Switching on and off transgene expression within lactotrophic cells in the anterior pituitary gland in vivo. Endocrinology 1422521-2532. [DOI] [PubMed] [Google Scholar]
- 43.Southgate, T., P. Kingston, and M. G. Castro. 2000. Gene transfer into neural cells in vivo using adenoviral vectors, p. 4.23.1-4.23.40. In C. R. Gerfen, R. McKay, M. A. Rogawski, D. R. Sibley, and P. Skolnick (ed.), Current protocols in neuroscience, vol. 4. John Wiley & Sons, New York, NY. [Google Scholar]
- 44.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12839-846. [DOI] [PubMed] [Google Scholar]
- 45.Virella-Lowell, I., B. Zusman, K. Foust, S. Loiler, T. Conlon, S. Song, K. A. Chesnut, T. Ferkol, and T. R. Flotte. 2005. Enhancing rAAV vector expression in the lung. J. Gene Med. 7842-850. [DOI] [PubMed] [Google Scholar]
- 46.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 837-44. [DOI] [PubMed] [Google Scholar]
- 47.Xiong, W., S. Goverdhana, S. A. Sciascia, M. Candolfi, J. M. Zirger, C. Barcia, J. F. Curtin, G. D. King, G. Jaita, C. Liu, K. Kroeger, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 8027-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu, Z. L., H. Mizuguchi, T. Mayumi, and T. Hayakawa. 2003. Woodchuck hepatitis virus post-transcriptional regulation element enhances transgene expression from adenovirus vectors. Biochim. Biophys. Acta 1621266-271. [DOI] [PubMed] [Google Scholar]
- 49.Zermansky, A. J., F. Bolognani, D. Stone, C. M. Cowsill, G. Morrissey, M. G. Castro, and P. R. Lowenstein. 2001. Towards global and long-term neurological gene therapy: unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol. Ther. 4490-498. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3697-707. [DOI] [PubMed] [Google Scholar]
- 51.Zirger, J. M., C. Barcia, C. Liu, M. Puntel, N. Mitchell, I. Campbell, M. Castro, and P. R. Lowenstein. 2006. Rapid upregulation of interferon-regulated and chemokine mRNAs upon injection of 108 international units, but not lower doses, of adenoviral vectors into the brain. J. Virol. 805655-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zufferey, R., J. E. Donello, D. Trono, and T. J. Hope. 1999. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 732886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.